Retraction Watch readers are likely familiar with the U.S. Office of Research Integrity (ORI), the agency that oversees institutional investigations into misconduct in research funded by the NIH, as well as focusing on education programs.
Earlier this month, ORI released data on its case closures dating back to 2006. We've charted those data in the graphics below. In 2021, ORI made just 3 findings of misconduct, a drop from 10 — roughly the average over the past 15 years — in 2020. Such cases can take years.
As the first chart makes clear, a similar dip in ORI findings of misconduct occurred in 2016. That was then-director Kathy Partin's first year in the role, and a time of some turmoil at the agency. In an interview with us then, Partin referred multiple times to the agency being short-staffed. Partin was removed from the post in 2017 and became intramural research integrity officer at the NIH in 2018.
ORI — as has often been the case over the past two decades — is once again without a permanent director. The most recent permanent director, Elisabeth (Lis) Handley, became Principal Deputy Assistant Secretary for Health in the Office of the Assistant Secretary for Health in July 2021.
We asked ORI to explain what's behind the figures. A spokesperson responded on their behalf.
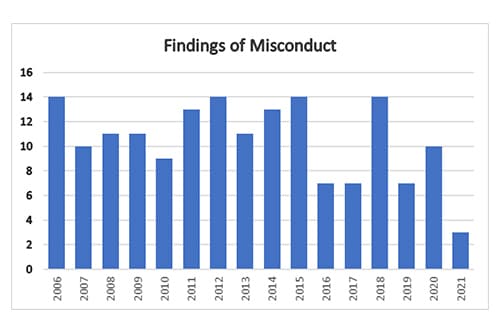
What is ORI's explanation for the fact that there were so few findings in 2021?
After ORI conducts a thorough oversight review for cases in which the institution has submitted its final investigation report, ORI may or may not concur with the institutional findings (that research misconduct occurred or did not occur). ORI also must decide whether to pursue or decline to pursue (DTP) separate administrative actions. ORI may conclude that the misconduct lacks significance (e.g., no published papers or grants) or that PHS funds were not involved and closes the case as a DTP. ORI's DTP closure of a case is not an exoneration of the respondent, and an institution can implement its own administrative actions based on its determination of the respondent's research, scientific, or professional misconduct. In CY 2021, ORI closed 41 cases (Finding of Research Misconduct, No Finding of Research Misconduct, and DTP), compared to 48 in CY 2020 (Finding of Research Misconduct, No Finding of Research Misconduct, and DTP).
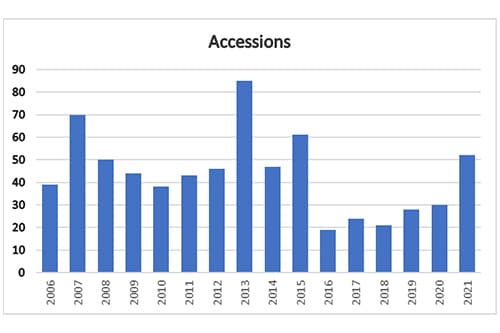
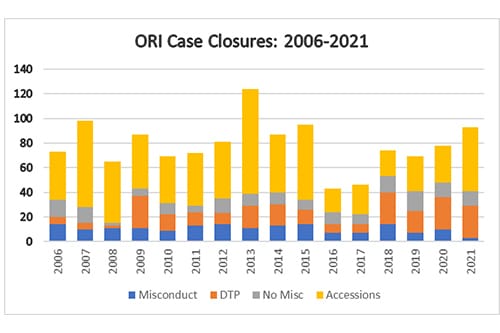
Per the ORI, and a declined to pursue (DTP) closure "involves a case in which an institution found that research misconduct occurred and may implement administrative actions against the respondent, but during its oversight review, ORI determined that a separate PHS finding of research misconduct was not warranted." A "no-misconduct closure involves a case in which the institution conducted an investigation and determined that research misconduct did not occur. Based on a preponderance of the evidence during its oversight review, ORI concurred with the institution's determination." And an accession "involves a case that was resolved during the assessment or inquiry stage of the institutional proceeding. Generally, during its assessment or inquiry into the allegation(s), the institution determined that there was insufficient evidence to warrant proceeding to an investigation. Based on its subsequent review of the institution's assessment or inquiry report, ORI concurred with the institution." Accessions include cases in which ORI decided the case was outside of its jurisdiction.
What is ORI's explanation for why accessions nearly doubled, but findings declined by more than half?
As nearly every labor sector has experienced, institutional workflows were altered over the past two years of COVID-related building closures and staffing issues. These closures in part affected the ability of some institutions to complete their proceedings within the time limitations as specified in the federal regulations. Although the number of allegations received over that time has changed little, the delays in receiving completed institutional reports meant that ORI could focus on the full range of potential accession closures. ORI notes that some allegations may involve many published papers or grant applications with multiple respondents from various institutions, further enumerating the complexity of a case and completing its review. The number of accessions for any calendar year does not necessarily reflect the year in which ORI received the allegation, when the institution started or completed its research misconduct proceeding, or when ORI initiated its oversight review. Generally, an accession closure involves an institutional proceeding that did not progress to an investigation, and ORI's oversight review concurred with the institution's determination that there was insufficient evidence to warrant proceeding to an investigation. In other cases, ORI may not have jurisdiction (does not involve PHS funded-research or is outside the 42 C.F.R. Part 93 definition of research misconduct). ORI would close such a case while the institution proceeds to an investigation under its own (or other funding agency's) authority and relevant regulations. ORI closed 52 accessions in CY 2021 after thorough oversight review of the associated allegations and institutional outcomes. The increase in accession closures reflects tireless work carried out at ORI and by institutions.
Is ORI concerned about how long cases typically take to be adjudicated?
ORI recognizes the importance of and is focused on fully addressing allegations of potential research misconduct in the most effective and efficient way possible in accordance with 42 C.F.R. Part 93. ORI also recognizes that ensuring due process and a full, fair, and independent examination of allegations is in the best interest of all involved. It is important to remember that institutional processes take time. Sometimes the investigation must be expanded in scope to consider other possible research misconduct committed by the same or additional respondents or to examine additional papers and grant applications that were not part of the initial allegations. A thorough oversight review, which ORI undertakes when the institutions complete their work, also takes time. ORI hopes to expand its use of technology for file submission, reporting of allegations, and information processing to improve overall processes, efficiencies, and case closure rates in the coming years.
The Center For Scientific Integrity
The mission of the Center for Scientific Integrity, the parent organization of Retraction Watch, is to promote transparency and integrity in science and scientific publishing, and to disseminate best practices and increase efficiency in science.
The goals of the Center fall under four broad areas:
- A database of retractions, expressions of concern and related publishing events, generated by the work of Retraction Watch. The database will be freely available to scientists, scholars and anyone else interested in analyzing the information.
- Long-form, larger-impact writing, including magazine-length articles, reports and books.
- Scholarship on scientific integrity and incentives in science.
- Aid and assistance to groups and individuals whose interests in transparency and accountability intersect with ours, and who could benefit from shared expertise and resources.
The Center is a 501(c)3 non-profit. Its work has been funded by generous grants from the John D. and Catherine T. MacArthur Foundation, the Laura and John Arnold Foundation, and the Leona M. and Harry B. Helmsley Trust.
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.