On April 19, the Japanese health ministry officially authorized the coronavirus vaccine developed by US pharmaceutical company Novavax Inc. to be used for people aged 18 and older. It is the fourth vaccine made available in the country, following mRNA vaccines made by Pfizer and Moderna and AstraZeneca's viral vector vaccine. The ministry is expected to start administering the Novavax vaccine in late May at the earliest.
Protein-based
The Novavax shot is what is known as a "recombinant protein vaccine." The chemical process involves an artificially made spike protein stimulating an immune response in the recipient. Similar vaccines are widely used for people with shingles and type-B hepatitis, among other viruses.
The ministry hopes to administer the vaccine to people who are allergic to the existing vaccines. Protein-based vaccines are known to cause allergic reactions with less frequency.
Two-dose vaccine and booster
Like the mRNA options, the Novavax vaccine is a two-dose shot with an interval of about three weeks. The ministry plans to make a booster shot available at least six months after the second dose. It is asking prefectural governments to open at least one facility for the administration of the Novavax vaccine. The shot must be stored and transported at temperatures between 2 and 8 degrees Celsius. They can be stored in standard-use refrigerators. Unused doses remain effective for nine months.
Manufactured in Japan
Though it was developed by an American company, the Novavax vaccine will be produced in Japan at the Takeda Pharmaceutical Co. factory in Yamaguchi Prefecture. The company plans to produce about 150 million doses within a year.
The ministry says the government arranged the purchase of the vaccine last September to ensure there would be a stable supply in the event of possible export restrictions on overseas vaccines. The ministry says it chose the Novavax vaccine both because of the strong track record of protein-based shots and to offer people a variety of options.
According to Novavax, its vaccine has been approved for conditional sale in the EU and UK. It has not been approved in the US, though the company did apply for emergency use authorization with the Food and Drug Administration earlier this year.
Effectiveness
On December 15, 2021, the New England Journal of Medicine published a clinical study on the vaccine by Novavax, US universities, and various medical organizations. It targeted about 30,000 people aged 18 and older in the US and Mexico between December 2020 and February 2021. It found the vaccine's effectiveness at preventing symptoms three weeks after the second dose was 90.4 percent, while its effectiveness at preventing medium and severe illness was 100 percent. At the time of the study, the Alpha and Beta variants had spread widely. The vaccine prevented symptoms in 92.6 percent of cases of these strains.
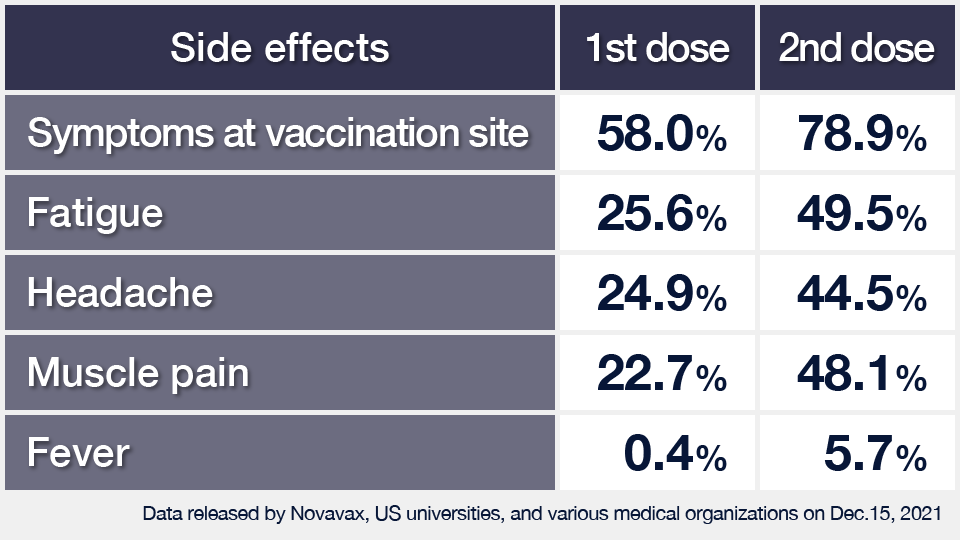
Side effects less common than with other vaccines
The study found that side effects subside within one or two days in most cases. They were also reported less often than after other vaccines and there were no confirmed cases of heart muscle inflammation or thrombosis.
"Good to have alternative"
"The Novavax vaccine is manufactured with the same method as vaccines that were used before the pandemic," says Professor Nakayama Tetsuo, a virologist with Tokyo's Kitasato University. "This means people may feel it is safer than the others."
"It is good to have another alternative to the mRNA vaccines," he adds. "Some people are hesitant to receive mRNA vaccines, due to side effects. This has led to a delay in the rollout of the third shot. The Novavax vaccine is believed to lead to side effects with less frequency so I think it will help increase the percentage of people with third shots."
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.