UroGen Pharma announced findings from a secondary analysis from the pivotal Phase 3 OLYMPUS trial which showed that UGN-101 for instillation, an investigational mitomycin formulation, demonstrated a 59% complete response rate in a subset of patients with endoscopically unresectable low-grade upper tract urothelial cancer. The analysis showed that in the OLYMPUS intent-to-treat population, 71 patients had undergone PDE at the time of the analysis and 42 of the 71 patients achieved a CR. 41 patients entered follow-up. Of the evaluated complete responses to date, 27 patients have undergone a six-month evaluation, and 24 out of 27 patients have remained disease free at six months. Overall, 5 of 41 patients who achieved a CR have relapsed at any time during the study. Of these 71 patients, 34 were initially characterized by the treating physician as having endoscopically unresectable tumor at baseline, and 20 of 34 of these patients achieved a CR at the PDE. The most common adverse events observed were urinary tract infection, ureteral narrowing and stricture formation. The majority of ureteral events were reported as mild to moderate and have resolved. The company initiated its rolling submission of the UGN-101 New Drug Application to the U.S. Food and Drug Administration in December 2018. The FDA previously granted Orphan Drug, Fast Track, and Breakthrough Therapy Designations to UGN-101 for the treatment of UTUC. If approved, UGN-101 would be the first drug approved for the non-surgical treatment of LG UTUC
Search This Blog
Sunday, May 5, 2019
Ionis, which has transformed medicine, undergoes its own transformation
Three decades after Ionis Pharmaceuticals was founded, the Carlsbadbiotech company is going through its first change of leadership.
Stanley T. Crooke, the only CEO Ionis has ever known, steps down in January to become executive chairman. Crooke, 73, will be replaced by another Ionis veteran, chief operating officer Brett Monia, 57.
The transformation during these 30 years has been huge.
When Crooke founded what was then Isis Pharmaceuticals, the company had just a handful of employees. It had no products. And even when its drugs began reaching the market, Ionis kept racking up heavy losses.
Today, Ionis is becoming a top-tier biotech company. With a growing number of drugs approved and nearing approval, its market value recently exceeded $11 billion for the first time. (It has since dropped back to about $10 billion, largely due to positive news from a competitor.)
And it now foresees continuous and growing profits as its drugs reach patients.
Ionis’ most successful drug, Spinraza, halts a wasting disease called spinal muscular atrophy. Marketed by Biogen, Spinraza brought in $1.72 billion in 2018. Biogen, which sells the drug, paid Ionis nearly $240 million in royalties.
Another Ionis drug, for Huntington’s disease, has shown early signs in experimental human testing that it may be effective against the invariably fatal illness.
Many Ionis employees and collaborators at academic centers such as UC San Diego made this possible. But Crooke has been the single most essential person to the success of Ionis, said John McCamant, editor of the Medical Technology Stock Letter in Berkeley.
“The story’s about Stan,” McCamant said. “It’s his company, his patents, the whole deal.”
The Medical Technology Stock Letter, a company then led by his father Jim McCamant, recommended buying Ionis stock in its 1991 initial public offering.
This transformation was driven by a drug technology Ionis pioneered called antisense. The technology blocks or modifies production of proteins involved in diseases.
Antisense drugs intercept specific sequences of RNA, which bring to cells the genetic instructions encoded in DNA. Complementary molecules Ionis calls “designer DNA” bind to the targeted RNA. This can suppress the effect of a certain gene or change how it works.
From the beginning, Crooke was known as antisense’s staunchest advocate. In the late 1980s, when antisense was strictly a laboratory tool, Crooke dreamed of turning that technology into a new platform to make genetically targeted drugs.
Turning that dream into reality required answering a number of hard questions. How to produce stable molecules? How to safely administer them? How to get them into cells in therapeutic doses? Which RNA molecules are the best targets for each disease?
The answers, discovered over the lifespan of Ionis, are documented in the patents Ionis holds.
Different styles
Monia, the incoming CEO, has led the company’s programs in oncology and rare diseases, the latter of which yielded Spinraza.
Another rare disease drug, Tegsedi, treats hereditary transthyretin-mediated amyloidosis, which causes nerve damage. FDA approval of Tegsedi was announced in October.
Both Monia and Crooke began as academics before crossing over into industry. However, they have different styles.
“Stan is a much more direct individual than I am,” Monia said. “He’s very detail-oriented. I tend to step back a little bit and delegate more to my individuals, but monitor very closely and jump in if I feel like I need to.”
The differences aren’t an issue.
“He has said multiple times that’s fine,” Monia said. “There’s no one way to manage an organization and it might be time for a new approach.”
As executive chairman, Crooke’s role will be a “strategic adviser,” looking for the long-term view, Monia said.
Monia said he understands the challenge ahead.
“I’ve learned a great deal from him and we have common core principles that we follow for the organization. However, I don’t plan on walking in Stan’s shoes. I brought my own shoes.”
The transition began a few years ago with conversations between Crooke and the Ionis executive team, including Monia. The conversation was broadly centered on preparing a succession plan.
“He got a lot of input from the executive team, including me,” Monia said. “And the conversations became less of group discussions (and) more sort of individual discussions that I started having with him.”
It was agreed that whoever succeeded Crooke should know antisense well, and should know the company’s history. And since Monia fit that picture, Crooke encouraged him to throw his hat into the ring.
To prepare for the CEO role, Monia became chief operating officer at the beginning of 2018. This job required Monia, trained as a drug research and development scientist, to become more familiar with the financial aspects of Ionis.
“I had to make sure that this was an aspect of the business that I liked,” Monia said. “Toward the end of last year it started coming together.”
Monia’s succession was formally announced in December, starting the year-long transition.
Mentor
For Monia, leading Ionis caps a career that he decided at the outset would center on discovering and developing new drugs.
Monia prepared by getting degrees in molecular biology and analytical chemistry at Stockton State College in New Jersey. He then got a Ph.D. in pharmacology at the University of Pennsylvania. There he met Crooke, who in addition to teaching also headed research at SmithKline Beckman.
Crooke impressed Monia with his multiple roles, teaching graduate-level course in pharmacology while handling worldwide research for a major pharmaceutical company.
“I decided that the best way for me to make an impact in the industry as rapidly as possible, would be to work with Stan,” Monia said. “We spent a lot of time together and talked about science and patients.”
Monia went on to earn his doctoral degree in Crooke’s laboratories.
By that time, Crooke had become fascinated by antisense. This technology allowed precise control over genetic activity, affecting only the desired genes while leaving others unaffected.
From his pharmaceutical experience, Crooke knew that even one drug could transform a company. He made that point in a May 1984 New York Times article about the stomach acid blocker Tagamet.
“SmithKline Beckman is in transition,” Crooke said in the article. “And that transition is driven by Tagamet. It’s hard to fathom, but the truth is this company is being changed by a drug.”
Five years later, Crooke was faced with even bigger possibilities. Antisense wasn’t just one drug, it was what pharma industry people call a platform technology that could give rise to a multitude of drugs.
Crooke decided antisense deserved its own company to exploit that potential. He founded Isis Pharmaceuticals, moved to San Diego and asked Monia to join his quest.
Carlsbad was a deliberate choice, because it had open space and was less expensive than San Diego’s biotech hub in La Jolla, Monia said.
The cross-country move was meant to be temporary.
“I told my mother I’ve got to come out here for five years, and then I’ll go back to the East Coast where my family was,” he said. “That obviously didn’t pan out.”
Ionis Pharmaceuticals Quotes by TradingView
By the numbers
A major selling point for a company like Ionis is the social benefit of treating severe diseases. But in strictly financial terms, has Ionis been a good stock? That depends on many factors, including how long ago the stock was purchased.
In general, those who bought within the last year, and for much of the last three years, should be happy. But those who bought at the $10 IPO price have seen mixed results. Their shares have increased in value, but massive dilution has taken even more.
The market capitalization of Ionis has increased by 100 fold since its IPO, from slightly over $100 million to more than 11 billion. That equals about 18 percent compound growth per year.
By comparison, during that 28-year period the Nasdaq index has increased from 500 to 7,600, or 15-fold
However, Ionis shares have been heavily diluted with repeated offerings — something normal for growing biotech companies. So that share purchased at the $10 IPO price didn’t increase nearly 100 times. Instead, per-share price has increased less than eight-fold.
So was the call to invest in Ionis made too soon? “That’s not an answerable question,” McCamant said. All biotech companies face setbacks from factors they don’t control.
For example, the first Ionis drug, for an AIDS-related eye infection, reached the market about the same time as the first effective HIV drugs. That dramatically lowered the market potential for the drug, Vitravene.
Ionis kept a focus on developing antisense and securing patents, McCamant said.
“One has to give them some odds of success when you have 35-plus drugs in the pipeline, with easily 10 new drugs coming into the market over the next five to 10 years,” McCamant said.
While Ionis has grown dramatically over the years, it still faces competition. Sales of its blockbuster Spinrase are likely to decline starting in 2020 as other drugs reach the market, wrote Morningstar analyst Karen Andersen in a Feb. 27 report.
“In addition, despite strong data in clinical trials to date and multiple partnerships, Ionis is just reaching sustainable profitability,” Andersen wrote.
As for Monia, Andersen wrote that his science background and seven years of executive experience at Ionis should serve him well.
Monia says he’s using the transition period to continue learning from Crooke about being a CEO.
“At the end of this year, I’ll essentially be doing this on my own,” Monia said.
Esperion announces FDA acceptance of NDAs for Bempedoic Acid, combo tablet
Esperion announced that the U.S. Food and Drug Administration has accepted both New Drug Applications for bempedoic acid and the bempedoic acid/ezetimibe combination tablet for filing and regulatory review. Bempedoic acid and the bempedoic acid/ezetimibe combination tablet were developed to be complementary, cost-effective, convenient, once-daily, oral therapies for the treatment of patients with elevated low-density lipoprotein cholesterol who need additional LDL-C lowering despite the use of currently accessible therapies. The PDUFA goal date for the completion of the bempedoic acid NDA review is set for February 21, 2020, and the PDUFA goal date for completion of the bempedoic acid/ezetimibe combination tablet NDA review is set for February 26, 2020. The FDA has communicated that there is no current plan to hold an advisory committee meeting to discuss the applications.
Cytokinetics Phase 2 Results in ALS Trial at American Academy of Neurology
Trial Did Not Meet Statistical Significance for Primary Efficacy Analysis
| ||||||||||||||||||||
Patients on All Doses of Reldesemtiv Declined Less Than Patients on Placebo
for SVC and ALSFRS-R, With Clinically Meaningful Differences Emerging Over Time
for SVC and ALSFRS-R, With Clinically Meaningful Differences Emerging Over Time
Early Terminations and Serious Adverse Events
Were Balanced Across Treatment Arms
Were Balanced Across Treatment Arms
Investor Event & Conference Call on May 6, 2019 at 7:30 a.m. Eastern Time
Conference Call/Webcast
Cytokinetics will host an investor event and conference call on May 6, 2019 at 7:30 a.m. Eastern Time including members of management and members of the Steering Committee of FORTITUDE-ALS. The event will be held at the Loews Philadelphia Hotelin the Lescaze Room. The conference call will be simultaneously webcast and will be accessible in the Investors & Media section of Cytokinetics’ website. The live audio of the conference call is also accessible via telephone to investors, members of the news media and the general public by dialing either (866) 999-2985 (CYTK) (United Statesand Canada) or (706) 679-3078 (International) and typing in the passcode 4284768. An archived replay of the webcast will be available via Cytokinetics’ website until June 3, 2019. The replay will also be available via telephone from May 6, 2019 at 11:00 a.m. Eastern Time until June 3, 2019 by dialing (855) 859-2056 (United States and Canada) or (404) 537-3406 (International) and typing in the passcode 4284768.
Cytokinetics, Incorporated (Nasdaq: CYTK, “Cytokinetics”) announced that results of FORTITUDE-ALS (Functional Outcomes in a Randomized Trial of Investigational Treatment with CK-2127107 to Understand Decline in Endpoints – in ALS) were presented today by Jeremy Shefner, M.D., Ph.D., Lead Investigator of FORTITUDE-ALS, Professor and Chair of Neurology at Barrow Neurological Institute, and Professor and Executive Chair of Neurology at the University of Arizona, Phoenix, at a podium presentation at the American Academy of Neurology Annual Meeting in Philadelphia.
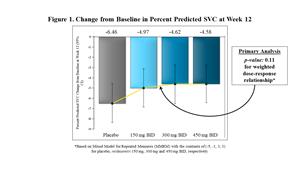
FORTITUDE-ALS did not achieve statistical significance for a pre-specified dose-response relationship in its primary endpoint of change from baseline in slow vital capacity (SVC) after 12 weeks of dosing (p=0.11). Similar analyses of ALSFRS-R and slope of the Muscle Strength Mega-Score yielded p-values of 0.09 and 0.31, respectively. However, patients on all dose groups of reldesemtiv declined less than patients on placebo for SVC and ALSFRS-R, with larger and clinically meaningful differences emerging over time.
While the dose-response analyses for the primary and secondary endpoints did not achieve statistical significance at the level of 0.05, in a post-hoc analysis pooling the doses together, patients who received reldesemtiv in FORTITUDE-ALS declined less than patients who received placebo. The trial showed effects favoring reldesemtivacross dose levels and timepoints with clinically meaningful magnitudes of effect observed at 12 weeks for the primary and secondary endpoints. The differences between reldesemtiv and placebo in SVC and ALSFRS-R total score observed after 12 weeks of treatment were still evident at follow-up, four weeks after the last dose of study drug.
The incidence of early treatment discontinuations, serious adverse events and clinical adverse events in FORTITUDE-ALS were similar between placebo and active treatment arms. The most common clinical adverse effects in the trial included fatigue, nausea and headache. The leading cause for early termination from FORTITUDE-ALS for patients who received placebo was progressive disease; the leading cause for early termination for patients who received reldesemtiv was a decline in cystatin C based estimated glomerular filtration rate (eGFR), a measure of renal function. Elevations in transaminases and declines in cystatin C eGFR were dose-related.
“Results from FORTITUDE-ALS are among the most impressive we have seen in a Phase 2 clinical trial in ALS,” said Dr. Shefner. “Especially noteworthy are the consistency and durability of effects observed across treatment arms on clinically meaningful endpoints.”
In collaboration with Astellas, Cytokinetics is developing reldesemtiv, a next-generation fast skeletal muscle troponin activator (FSTA), as a potential treatment for people living with debilitating diseases and conditions associated with skeletal muscle weakness and/or fatigue.
Essa Pharma presents new preclinical data for EPI-7386
ESSA Pharma presented new preclinical data on ESSA’s lead Investigational New Drug candidate at the 2019 American Urological Association Annual Meeting. The studies demonstrate that, pre-clinically, EPI-7386: Displays similar in vitro IC50 potency compared to the ‘lutamide class of antiandrogens in an in vitro androgen receptor inhibition assay; Shows in vitro activity in several enzalutamide-resistant prostate cancer cell models in which enzalutamide is resistant; Exhibits a favorable metabolic profile across three preclinical animal species, which suggests that EPI-7386 will have high exposure and a long half-life in humans; Provides similar antitumor activity to enzalutamide in the enzalutamide-sensitive LNCaP prostate cancer xenograft model; Provides superior antitumor activity to enzalutamide, as a single agent or in combination with enzalutamide, in the enzalutamide-resistant VCaP prostate cancer xenograft model; AR inhibition with both an N-terminal domain inhibitor and a ligand binding domain inhibitor, induces deeper and more consistent anti-tumor responses in the enzalutamide-resistant VCaP xenograft model.
Neurocrine presents data analysis from two Phase III studies of opicapone
Neurocrine announced the presentation of a data analysis from two Phase III studies of opicapone, a novel, once-daily, oral, selective, peripherally-acting catechol-O-methyltransferase inhibitor for the treatment of Parkinson’s disease. The analysis found that treatment with opicapone 50 mg, added to levodopa, resulted in a significant and sustained increase in ON time without troublesome dyskinesia, in Parkinson’s disease patients with motor fluctuations. In addition, more than 60% of patients treated with once-daily opicapone 50 mg achieved greater than or equal to a one-hour increase from baseline in total ON time at week 14/15. The analysis, which included data from more than 900 patients in the double-blind, placebo-controlled Phase III BIPARK-1 and BIPARK-2 studies, was highlighted as an oral session at the 2019 American Academy of Neurology Annual Meeting in Philadelphia. The data presentation highlighted statistically significant increases in absolute ON time without troublesome dyskinesia from baseline to the week 14/15 endpoint in both the BIPARK-1 and BIPARK-2 studies. The improvements in ON time without troublesome dyskinesia were sustained in all patients treated with opicapone in the one-year long-term open-label extension studies, with an average increase from baseline of 2.0+/-2.6 hours in BIPARK-1 and 1.8+/-3.2 hours for BIPARK-2. In addition, a significantly higher percentage of patients treated with opicapone 50 mg had an increase in total ON time of an hour or longer at week 14/15 in both BIPARK-1 and BIPARK-2. Pooled safety data from the double-blind opicapone-treated population showed that 17.4% patients treated with opicapone reported dyskinesia as a treatment-emergent adverse event versus 6.2% in placebo-treated patients. Only 1.9% of opicapone-treated patients discontinued treatment due to a TEAE of dyskinesia and only 0.3% experienced dyskinesia as a serious TEAE. Other TEAEs included constipation, insomnia and dry mouth in opicapone- and placebo-treated patients, respectively.
Need to model aging in a hurry? Do it in outer space
What do you do when you’re studying age-related diseases but can’t wait around for tissue models to reach a ripe old age? You blast them into outer space, naturally.
It’s no secret that after traveling in space, astronauts’ bodies experience changes that resemble aging: bone loss, muscle deterioration and altered immune systems, to name a few. These result from prolonged exposure to microgravity, or gravity that is “diminished or close to zero gravity compared with Earth.” Scientists at the National Institutes of Health (NIH) and the International Space Station (ISS) are harnessing these age-accelerating effects to create models of various age-related diseases, a process that includes sending tissue models into space.
Tissue chips—also called tissue-on-a-chip or organ-on-a-chip—are tiny 3D models of human organ systems. Scientists use them to model diseases and test how drugs might affect specific organs. The NIH struck a deal this week with StemoniX to use its brain-on-a-chip technology in opioid research.
“Tissue chips in space provide a way to model various diseases of the aging process. Such models can be difficult or take a long time to develop here on earth but are greatly facilitated under microgravity, and scientists can use them to develop drugs that can prevent or slow down those diseases,” said Danilo Tagle, Ph.D., associate director for special initiatives at NIH’s National Center for Advancing Translational Sciences (NCATS), in a statement. “Taking this technology into space is an unprecedented opportunity to use tissue chips for accelerating translational development of interventions for use here on earth to treat many aging-related diseases.”
NCATS, the NIH’s National Institute of Biomedical Imaging and Engineering, along with the ISS U.S. National Laboratory, are supporting research under their Tissue Chips in Space program. The first set of chips—developed at the University of California, San Francisco to model the immune system—arrived at the ISS in December. A second shipment is due to blast off this weekend.
That set includes lung and bone marrow chips from the Children’s Hospital of Philadelphia and University of Pennsylvania, kidney chips from the University of Washington, bone and cartilage chips from MIT and chips modeling the blood-brain barrier from the biotech company Emulate. The hope is these projects will speed up the development of treatments for osteoarthritis, kidney stones and other conditions.
The team on the space station will infect the lung and bone marrow chip with Pseudomonas aeruginosa, a bacterium associated with hospital-acquired diseases, said Lucie Low, Ph.D., the scientific program manager for the NIH’s Tissue Chip for Drug Screening program.
“They will see how the bacterium affects lung tissues—because we know that the immune system changes up in space—and they will also be looking at how the bone marrow responds to that lung infection by the mobilization of neutrophils, which are white blood cells, in the bone marrow,” she said.
The cartilage and bone chips will be modeling post-traumatic osteoarthritis in the knee joint.
“They will be looking at a lot of different kinds of ‘omics’ outcomes from the tissues when they are up in microgravity: proteomics, metabolomics, different kinds of cellular, molecular and metabolic changes in tissues that result from microgravity,” Low said.
The kidney chip, meanwhile, will help with osteoarthritis research, as well as research into kidney stones.
“Astronauts seem to suffer from an increase in kidney stones and lose a lot of bone mass very rapidly when they enter microgravity,” she said. “Because the kidney filters a lot of blood, an increased calcium concentration leached from the bones in microgravity turns up in the kidneys in the form of kidney stones.
The tissue chips are slated to hitch a ride from Cape Canaveral, Florida, this weekend—as part of a payload of more than 5,400 pounds that includes crew supplies, hardware and other scientific experiments. The launch was postponed twice this week due to technical issues, and is now scheduled for the early morning hours of Saturday.
Subscribe to:
Posts (Atom)