The yearly influenza season threatens to make the COVID-19 pandemic doubly deadly, but I believe that this isn’t inevitable.
There are two commonly given vaccines – the pneumococcal vaccine and the Hib vaccine – that protect against bacterial pneumonias. These bacteria complicate both influenza and COVID-19, often leading to death. My examination of disease trends and vaccination rates leads me to believe that broader use of the pneumococcal and Hib vaccines could guard against the worst effects of a COVID-19 illness.
I am an immunologist and physiologist interested in the effects of combined infections on immunity. I have reached my insight by juxtaposing two seemingly unrelated puzzles: Infants and children get SARS-CoV-2, the virus that causes COVID-19, but very rarely become hospitalized or die; and case numbers and death rates from COVID-19 began varying greatly from nation to nation and city to city even before lockdowns began. I wondered why.
One night I woke up with a possible answer: vaccination rates. Most children, beginning at age two months, are vaccinated against numerous diseases; adults less so. And, both infant and adult vaccination rates vary widely across the world. Could differences in the rates of vaccination against one or more diseases account for differences in COVID-19 risks? As someone who had previously investigated other pandemics such as the Great Flu Pandemic of 1918-19 and AIDS, and who has worked with vaccines, I had a strong background for tracking down the relevant data to test my hypothesis.
Pneumococcal vaccination rates correlate with lower COVID-19 cases and deaths
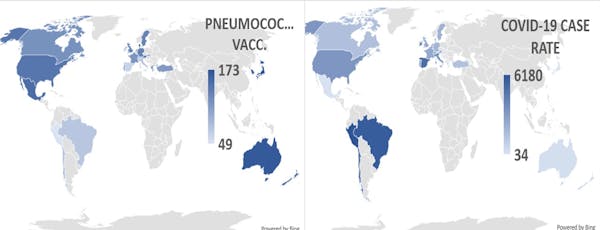
I gathered national and some local data on vaccination rates against influenza, polio, measles-mumps-rubella (MMR), diphtheria-tetanus-pertussis (DTP), tuberculosis (BCG), pneumococci and Haemophilus influenzae type B (Hib). I correlated them with COVID-19 case rates and death rates for 24 nations that had experienced their COVID-19 outbreaks at about the same time. I controlled for factors such as percentage of the population who were obese, diabetic or elderly.
I found that only pneumococcal vaccines afforded statistically significant protection against COVID-19. Nations such as Spain, Italy, Belgium, Brazil, Peru and Chile that have the highest COVID-19 rates per million have the poorest pneumococcal vaccination rates among both infants and adults. Nations with the lowest rates of COVID-19 – Japan, Korea, Denmark, Australia and New Zealand – have the highest rates of pneumococcal vaccination among both infants and adults.
A recent preprint study (not yet peer-reviewed) from researchers at the Mayo Clinic has also reported very strong associations between pneumococcal vaccination and protection against COVID-19. This is especially true among minority patients who are bearing the brunt of the coronavirus pandemic. The report also suggests that other vaccines, or combinations of vaccines, such as Hib and MMR may also provide protection.
These results are important because in the U.S., childhood vaccination against pneumococci – which protects against Streptococcus pneumoniae bacteria – varies by state from 74% to 92%. Although the CDC recommends that all adults 18-64 in high risk groups for COVID-19 and all adults over the age of 65 get a pneumococcal vaccination, only 23% of high-risk adults and 64% of those over the age of 65 do so.
Similarly, although the CDC recommends at all infants and some high-risk adults be vaccinated against Haemophilus influenzae type B (Hib), only 80.7% of children in the U.S. and a handful of immunologically compromised adults have been. Pneumococcal and Hib vaccination rates are significantly lower in minority populations in the U.S. and in countries that have been hit harder by COVID-19 than the U.S.
Based on these data, I advocate universal pneumococcal and Hib vaccination among children, at-risk adults and all adults over 65 to prevent serious COVID-19 disease.
How pneumococcal vaccination protects against COVID-19
Protection against serious COVID-19 disease by pneumococcal and Hib vaccines makes sense for several reasons. First, recent studies reveal that the majority of hospitalized COVID-19 patients, and in some studies nearly all, are infected with streptococci, which causes pneumococcal pneumonias, Hib or other pneumonia-causing bacteria. Pneumococcal and Hib vaccinations should protect coronavirus patients from these infections and thus significantly cut the risk of serious pneumonia.
I also found that pneumococcal, Hib and possibly rubella vaccines may confer specific protection against the SARS-CoV-2 virus that causes COVID-19 by means of “molecular mimicry.”
Molecular mimicry occurs when the immune system thinks one microbe looks like another. In this case, proteins found in pneumococcal vaccines and, to a lesser degree, ones found in Hib and rubella vaccines as well look like several proteins produced by the SARS-CoV-2 virus.
Two of these proteins found in pneumococcal vaccines mimic the spike and membrane proteins that permit the virus to infect cells. This suggests pneumococcal vaccination may prevent SARS-CoV-2 infection. Two other mimics are the nucleoprotein and replicase that control virus replication. These proteins are made after viral infection, in which case pneumococcal vaccination may control, but not prevent, SARS-CoV-2 replication.
Either way, these vaccines may provide proxy protection against SARS-CoV-2 infection that we can implement right now, even before we have a specific virus vaccine. Such protection may not be complete. People might still suffer a weakened version of COVID-19 but, like most infants and children, be protected against the worst effects of the infection.
Fighting influenza-related pneumonias during the COVID-19 pandemic
While the specific protection these other vaccines confer against COVID-19 has not yet been tested in a clinical trial, I advocate broader implementation of pneumococcal and Hib vaccination for one additional, well-validated reason.
Pneumococcal and Hib pneumonias – both caused by bacteria – are the major causes of death following viral influenza. The influenza virus rarely causes death directly. Most often, the virus makes the lungs more susceptible to bacterial pneumonias, which are deadly. Dozens of studies around the world have demonstrated that increasing rates of pneumococcal and Hib vaccination dramatically lowers influenza-related pneumonias.
Similar studies demonstrate that the price of using these vaccines is balanced by savings due to lower rates of influenza-related hospitalizations, intensive care unit admissions and deaths. In the context of COVID-19, lowering rates of influenza-related hospitalizations and ICU admissions would free up resources to fight the coronavirus, independent of any effect these vaccines might have on SARS-CoV-2 itself. In my opinion, that is a winning scenario.
In short, we need not wait for a SARS-CoV-2 vaccine to slow down COVID-19.
I believe that we can and should act now by fighting the coronavirus with all the tools at our disposal, including influenza, Hib, pneumococcal and perhaps rubella vaccinations.
Preventing pneumococcal and Hib complications of influenza and COVID-19, and perhaps proxy-vaccinating against SARS-CoV-2 itself, helps everyone. Administering these already available and well-tested pneumococcal and Hib vaccines to people will save money by freeing up hospital beds and ICUs. It will also improve public health by reducing the spread of multiple infections and boost the economy by nurturing a healthier population.