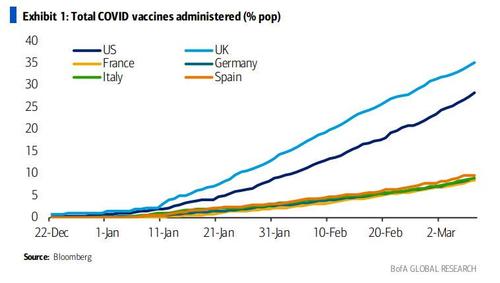
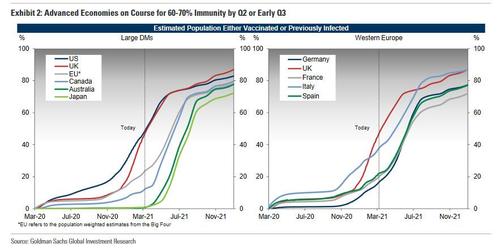
The recent failures of several potential Covid-19 therapies has reminded the world that, while vaccine development has gone remarkably smoothly so far, agents to treat the virus are still few and far between.
Merck & Co and Ridgeback will hope to change this with their oral antiviral molnupiravir, and it should not be long before it becomes apparent whether the project has a future: interim phase II/III data are expected this quarter, in hospitalised and non-hospitalised patients alike.
The companies did present some results on Saturday, but these were from a phase IIa study in non-hospitalised patients that primarily measured virologic efficacy – and, in a move that raised eyebrows, the groups only reported data on a secondary endpoint, time to viral negativity determined via isolation in Vero cell line culture.
Data on the primary endpoint, time to viral negativity measured using RT-PCR testing, will be reserved for an undisclosed upcoming medical meeting.
Still, the early data look promising: at day five none of the molnupiravir-treated patients showed evidence of the virus, compared with 24% of those on placebo, and there was evidence of a dose response. Merck and Ridgeback gave a nominal p value of 0.001 but noted that this had not been controlled for multiplicity.
However, Leerink analysts raised several questions, including the nature of three adverse events that led to drug discontinuations. Merck and Ridgeback said that, although the study remained blinded, none of the four serious adverse events seen in the trial were deemed related to molnupiravir.
Separately, Evercore ISI’s Umer Raffat noted that some virologists had expressed concerns about molnupiravir’s mutagenic mechanism. Merck and Ridgeback also said on Saturday that animal studies, in which the project was given for longer and at higher doses than in human trials, suggested that molnupiravir was not mutagenic or genotoxic.
Translating
Another big question is whether the latest data will translate into a benefit on harder endpoints such as reductions in hospitalisations and death. This is exactly what Merck’s upcoming trials, MK-4482-001 in the hospital setting and MK-4482-002 in the outpatient setting, are looking to show.
In hospitalised patients the bar for molnupiravir, albeit not a very high one, has been set by Gilead’s intravenous antiviral Veklury, which is FDA-approved despite fairly unimpressive results.
Handily, the MK-4482-001 trial has the same primary endpoint as that used in the Acct-1 study of Veklury, time to sustained recovery through 29 days. This should aid cross-trial comparisons, with the usual caveats.
In Acct-1 Veklury did significantly speed up recovery compared with placebo, but there was no statistically significant benefit on mortality.
| Veklury's pivotal results in Acct-1, hospitalised pts |
|---|
| Endpoint | Veklury | Placebo | p value |
Time to recovery within 29 days after*
randomisation | 10 days | 15 days | p<0.001 |
| 29-day mortality | 11% | 15% | NS |
| *Primary efficacy endpoint. Source: Veklury label. |
In non-hospitalised patients the more relevant comparator will be the monoclonal antibodies. Lilly’s bamlanivimab and its bamlanivimab plus etesevimab combo, and Regeneron’s casirivimab plus imdevimab, all have EUAs in the outpatient setting.
The primary efficacy endpoint of the MK-4482-002 trial of molnupiravir is the percentage of patients who are hospitalised or die.
| Data so far with the anti-Covid-19 MAbs |
|---|
| | Blaze-1 bamlanivimab alone | Blaze-1 bamlanivimab + etesevimab | Ph2/3 trial of casirivimab + imdevimab* |
| | Bamlanivimab | Placebo | Bamlanivimab + etesevimab | Placebo | Casirivimab + imdevimab | Placebo |
| Hospitalisations/deaths | 1.6% | 6.3% | 2.1% | 7.0% | 3% | 9% |
| *Hospitalisations and ER visits only. Source: FDA & company releases. |
If Merck can get a result with molnupiravir it will mark some rare good news in the antiviral space, which saw another failure this weekend in the shape of Abivax’s ABX464. This was said to have both antiviral and anti-inflammatory properties, but late on Friday Abivax disclosed that the pivotal Mir-Age study, which included hospitalised and non-hospitalised patients, had been stopped for lack of efficacy.
There are a few other antivirals left in development for Covid-19, but Merck and Ridgback’s is by far the most advanced.
| Antivirals in development for Covid-19 |
|---|
| Project | Company | Setting | Note |
| Veklury (IV) | Gilead | FDA-approved in Covid-19 patients requiring hospitalisation | Repurposed Ebola research project |
| Molnupiravir (oral) | Merck & Co/Ridgeback | Ph2/3 trials in hospitalised (NCT04575584) and non-hospitalised (NCT04575597) pts ongoing | Repurposed flu antiviral |
| AT-527 (oral) | Roche/Atea | Ph2 trial in hospitalised patients (NCT04396106) ongoing; non-hospitalised trial due to start (NCT04709835) | Repurposed hep C antiviral |
| PF-07304814 (IV) | Pfizer | Ph1b ongoing in hospitalised patients (NCT04535167, excludes severely ill or with certain pre-existing conditions) | Repurposed SARS research project |
| MP0420 (ensovibep; IV) | Novartis/Molecular Partners | Ph1 trial in UK ongoing in healthy volunteers | Designed for SARS-Cov-2 |
| MP0423 | Novartis/Molecular Partners | Preclinical | Designed for SARS-Cov-2; could be better against variants than MP0420 due to different targeting mechanisms |
Source: EvaluatePharma & clinicaltrials.gov.
https://www.evaluate.com/vantage/articles/news/trial-results/molnupiravirs-big-day-draws-near |