The CCC19 study included 928 patients with various types of cancers. In contrast, the TERAVOLT study included 400 patients with thoracic malignancies only. Another important difference was that in the CCC19, all patients had confirmed Real-time PCR COVID-19 infection, while in the TERAVOLT study, patients had either confirmed COVID-19 infection or had clinically suspected or radiologically suspected infection.
Dr. Curigliano also assessed the mortality rate in the CCC19 study. With a median of 21 days of follow up, 121 patients — representing 13% of the entire cohort — had died. In the TERAVOLT study, the median follow-up was 33 days, and 35.5% had died. There are many hypotheses for why patients with thoracic malignancies have a higher death rate, but one of these hypothesis states that these patients are prone to develop microvascular obstruction of lung vessels with the formation of microclots, manifesting as the thrombo-inflammatory syndrome (Figure 1).
Figure 1 – Thrombo-inflammatory syndrome:
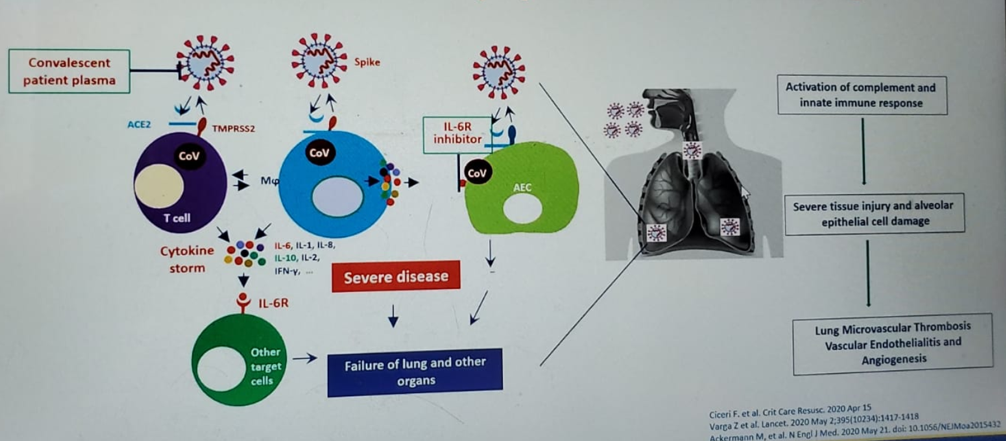
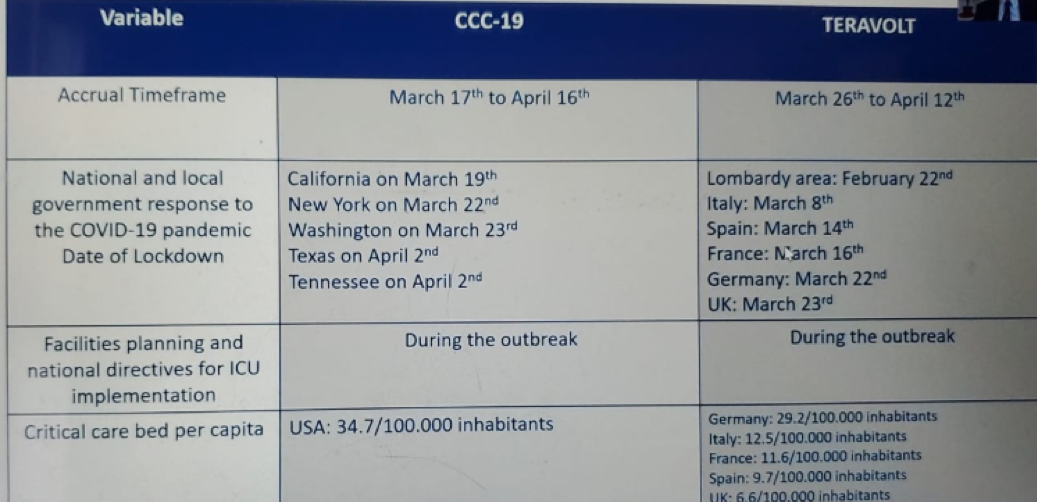
Table 1 compares the morbidity and mortality in the TERAVOLT and CCC19 studies and demontrates a higher mortality and hospitalization rate in the TERAVOLT study, but a lower ICU admission rate, and rate of mechanical ventilation. Table 2 demonstrates the independent factors for mortality in both studies, showing very similar risk factors. Table 3 shows important data as it presents the main differences between the two studies, including smoking status, gender, previous steroid use, cancer stage, and the region of patient residence. The data shown in Table 4 attempts to explain some of the presented differenced between these studies, which include the different accrual timeframe, the type of national and local government response to the COVID-19 pandemic, and the rate of critical care bed per capita, which was different in a staggering manner.
Table 1 – Morbidity and mortality in the TERAVOLT and CCC19 studies:

Table 2 – independent factors for mortality in the TERAVOLT and CCC19 studies:
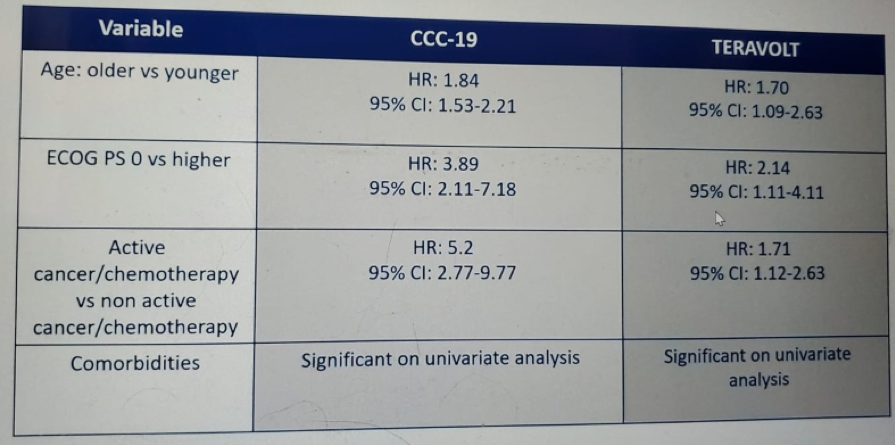
Table 3 – CCC19 and TERAVOLT main differences:
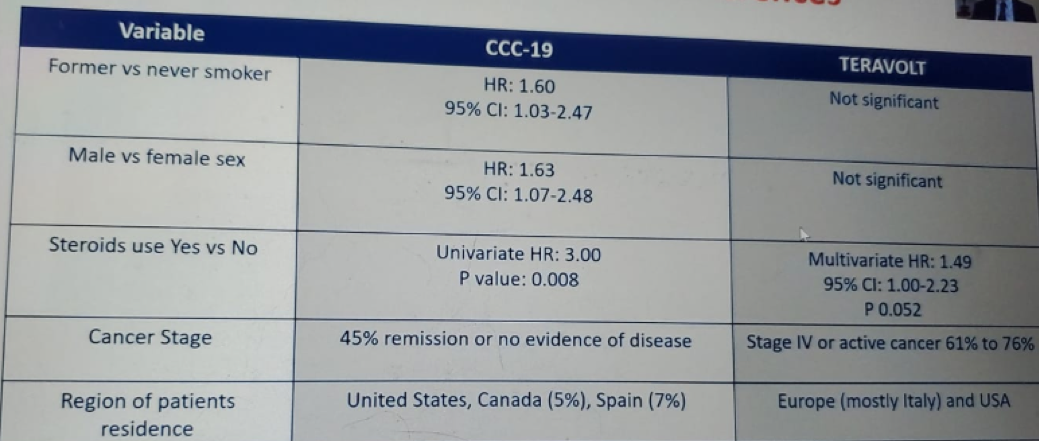
Table 4 – CCC19 and TERAVOLT possible explanations for the major differences between the studies:
Dr. Curigliano moved on to discuss the mortality rates of COIVD-19 in cancer patients in areas that were hit with COVID-19 quite substantially. The mortality rate of patients with cancer and COVID-19 in New York City was markedly increased compared to non-cancer patients, and compared to all New York City COVID-19 patients. The mortality rate in cancer patients with COVID-19 was significantly higher when compared to all patients with COVID-19 in New York City (25% vs. 14%). Specifically, there was a 50% mortality rate among lung cancer patients, 40% mortality rate for breast cancer patients, 50% mortality rate for genitourinary cancer patients, and 38% mortality rate for gastrointestinal cancer patients.
When assessing the case fatality rate in cancer patients in Milan, Italy, cancer patients demonstrated markedly increased COVID-19 mortality rate compared to non-cancer patients admitted to the ICU. The case fatality rates in cancer patients with COVID-19 was 19% in elderly patients )>75 years old), while the overall mortality rate in cancer patients was 8%.
Dr. Curigliano moved on to discuss the major limitations of both the TERAVOLT and CCC19 studies. These included:
- COVID-19 case definition in the TERAVOLT trial that was not standardized.
- In the European centers the pandemic scenario resulted in very difficult triage decisions (TERAVOLT study).
- The patient selection in the TERAVOLT study was problematic as well.
- There was a high proportion of stage four non-small cell lung carcinoma in the TERAVOLT study.
- In contrast, there was a high proportion of patients in remission in the CCC19 study.
- There was no data presented on the occurrence of deep vein thrombosis or pulmonary embolism in both studies
- There was no clear definition for eligibility to ICU.
- Both studies were cross-sectional studies with no real-time data capture, no auditing, and no standardized outcome definitions.
- In both studies, there are multiple biases, including selection bias, recall bias, confounding by indication, and changes in practice and or disease evolution.
- Lastly, there was no propensity score adjustment, no control with non-cancer patients, and there was no stratification for the stage and lines of previous therapies.
- In both studies, serious confounding may remain due to the impact of unmeasured confounders and unmeasured risks on treatment decisions.
There are still many questions that remain regarding cancer care prioritization and care intensity optimization. It is not clear if cancer patients should be prioritized for the ICU triage for admission based on the disease stage. It is also unclear whether immunotherapy provides any kind of protective effect from COVID-19 infection and outcome.
In conclusion, older age, increased number of comorbidities, worse performance status, active cancer, and chemotherapy alone or in combination with other treatments, have been shown to increase the risk of mortality in cancer patients with COVID-19. It is important for centers to prepare for the treatment not only of suspected and confirmed cases of COVID-19, but also to consider the treatment of cancer patients with COVID-19 and prepare accordingly. Lastly, cancer treatment prioritization should balance interventions based on the magnitude of benefit in the clinical setting.
Before ending his talk, Dr. Curigliano discussed some research priorities in cancer patients. According to him, it is important to understand the utility of PCR testing in these patients. Longitudinal serological studies are urgently needed to determine the extent and duration of immunity to COVID-19. It is critical that we develop an epidemiological model to estimate the cumulative incidence of COVID-19 within a specific timeframe and pandemic scenario. We need to identify viral, environmental, and immunological factors that, in combination, will determine the dynamics of COVID-19. It is also critical to determine the morbidity and mortality associated with COVID-19 according to the specific treatment that is given to cancer patients (chemotherapy, targeted therapy, and immune checkpoint blockade).
To conclude his talk, Dr. Curigliano reiterated three important principles that continue to guide us through this pandemic:
- Transparency is the best policy.
- During this pandemic, there was an unprecedented collaboration among clinicians and scientists globally to work together and generate data for patients with cancer.
- Surprisingly, 21st-century science played a relatively small role in dealing and controlling the COVID-19 pandemic, while 19th-century methodology continued to prove it’s value, with early implementation of social distancing, personal protective equipment, and handwashing.
https://www.urotoday.com/conference-highlights/asco-2020/asco-2020-prostate-cancer/121908-asco-2020-looking-back-on-covid-19-in-order-to-move-forward.html
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.