There are just nine days left in the year, and this year has flown by.
We've delivered readers a steady stream of 2026 outlooks over the past few days and weeks, and next up is a year-ahead catalyst list from Goldman Sachs' small-cap biotech stock coverage.
Goldman analysts, led by Corinne Johnson, held their third "Year-Ahead" Catalyst Clinic, highlighting significant clinical catalysts for small-cap biotech companies (market caps under $3 billion) in their stock coverage universe.
Featured companies in the Catalyst Clinic included AbCellera Biologics, Allogene Therapeutics, Apogee Therapeutics, BioAge Labs, Entrada Therapeutics, Gossamer Bio, Gubra, Lyell Immunopharma, Recursion Pharmaceuticals, Sana Biotechnology, Sionna Therapeutics, Sera Prognostics, Tyra Biosciences, Viralgen, and Viridian Therapeutics.
Johnson said the sentiment from the event was mostly positive, with several names showing clearer paths to value-creating data in 2026.
Here are the key catalysts across Goldman's small-cap biotech coverage, spanning immunology, oncology, metabolic disease, rare disease, and cell and gene therapy. Across the group, management teams highlighted upcoming Phase 1 and Phase 2 readouts, pivotal trial starts, and regulatory milestones:
AbCellera (ABCL): Ahead of proof of concept data from the Ph1 study (expected in mid-26), management underscored the vast opportunity for ABCL635, the company's most advanced wholly-owned asset under development for moderate-to-severe VMS associated with menopause. In particular, management discussed the benefits of an antibody approach (vs. small molecules) to targeting neurokinin 3 receptor (NK3R, which has been implicated in its role in neuroendocrine regulation and reproductive function), confidence in the ability of an antibody to cross the BBB and reach the receptor which sits on KNDy neurons in the hypothalamus (a key question for investors), and its expectations for a potentially differentiated profile per improved efficacy, safety/tolerability, and more attractive dosing regimen.
Allogene Therapeutics Inc. (ALLO): The discussion centered on the interim analysis of the pivotal ALPHA3 study of CD19- targeted cema-cel in frontline consolidated large B-cell lymphoma, and initial clinical data from the Ph1 RESOLUTION study of CD19/CD70 dual-targeted ALLO-329 in autoimmune indications. On the former, management views the ~30% bar for the delta in minimal-residual disease (MRD) conversion rate vs. the observation arm as achievable and de-risking to the primary endpoint of event-free survival. Further, management highlighted the potential ~$5bn opportunity, aided by increasing utilization of CAR-T therapies in the community setting and awareness of MRD testing. On the Ph1 RESOLUTION study, management discussed the goal of establishing proof-of-concept and providing insights on the ability to eliminate lymphodepletion preconditioning.
Apogee Therapeutics Inc. (APGE): We hosted APGE, where a catalyst-rich 2026 could potentially validate the product-in-a-pipeline potential for lead asset APG777 (anti-IL13). Management highlighted: 1) Ph1b data in mild-to-moderate asthma in 1Q26, where APGE seeks to demonstrate a Dupixent (anti-IL4Ra)-like profile, to support expansion into respiratory indications in combination with anti-TSLP (APG273); 2) Ph2 readouts in moderate-to-severe atopic dermatitis, where management expects to validate 3QM/6QM dosing in the Part A maintenance study (data in 1Q26), and further interrogate exposure-response and inform dose selection for Ph3 (initiation in 2H26) in Part B (data in 2Q26); and 3) Ph1b proof-of-concept data evaluating the OX40L combination APG279 against Dupixent in 2H26, where management expects to demonstrate deeper and broader responses in a more heterogeneous patient population.
BioAge Labs (BIOA): The discussion was focused on: (1) key 2026 catalysts, including additional Ph1 MAD data in 1H26 and Ph2a 12 week proof-of-concept data by YE26 in obese and inflamed patients to validate BGE-102's (oral CNS-penetrant, NLRP3 inhibitor) impact on hsCRP reduction, although management views weight loss benefit as pure upside; (2) BGE-102's potential in cardiovascular (CV) risk, where BIOA expects the NLRP3 class to be positioned as oral IL6 inhibitors, given the robust hsCRP reduction (~80%) seen with VTYX's asset earlier this year and in three patients with elevated baseline hsCRP treated with BGE-102 in Ph1 - albeit, BIOA seeks partnership to advance BGE-102 in CV risk; and (3) other prioritized indications for BGE-102 to be disclosed in the near-term, with feasible development pathways for a small biotech, where BIOA looks to establish differentiation leveraging BGE-102's strong penetration into the brain and retina.
Entrada Therapeutics Inc. (TRDA): Management discussed: 1) potentially best-in-class efficacy with 40% dystrophin production after a single injection versus del zota's ~10-11%, 2) preferential safety given efficient excretion of the oligonucleotide components, thereby circumventing hypomagnesemia, and 3) greater drug efficiency at lower dosing levels supported by a smaller carrier size.
Gossamer Bio Inc. (GOSS): Management discussed: 1) differentiation of its TKI seralutinib versus historical comparators given improved tolerability from systemic drug elimination and potential for disease remodeling, 2) the company's focus on an enriched population and statistical plan following learnings from the Phase 2 TORREY study, and 3) continued confidence in the regulatory outlook given consistency/alignment in interactions with the FDA.
Gubra A/S (GUBRA.CO): Key takeaways from the discussion: 1) Gubra see potential for ABBV-295 (long-acting amylin) to perform in line with eloralintide in terms of efficacy, safety and tolerability, 2) management see ABBV-295's c.11 day half life and extended Cmax as supportive of a monthly dosing regimen, and 3) Gubra see ABBV-295 being used as both a monotherapy and in combination with a partner.
Lyell Immunopharma (LYEL): The discussion focused on ronde-cel (autologous CD19/20-targeting CAR T-cell therapy) in relapsed/refractory large B-cell lymphoma (LBCL), including: (1) the longer-term Ph1/2 update at the American Society of Hematology (ASH) annual meeting, where the data continues to track competitively vs. approved CD19-targeted CAR T-cell therapies; and (2) pivotal trial strategy, where LYEL aims to demonstrate superiority vs. approved assets in the H2H study in 2L patients (enrollment initiation in early-2026), albeit approval is first expected in the 3L+ setting basis the PiNACLE study (final data in mid-2027, 2028 launch). Separately, LYEL also touched upon LYL273 in 3L+ metastatic colorectal cancer, where they expect further derisking per Ph1 updates in 2026 to support a pivotal start in 2027.
Sana Biotechnology (SANA): Management and discussed the company's key programs, where, post the recent pipeline re-prioritization, the focus is on: (1) SC451, its HIP-modified stem cell-derived pancreatic islet cell therapy for type 1 diabetes (T1D) - where SANA highlighted alignment with regulators regarding the GMP master cell bank and the path to an Investigational New Drug Application (IND) and Ph1 start as early as 2026 (noting the potential for initial data in 2026), and (2) SG293, an in vivo CAR T with CD8-targeted fusogen delivery of a CD19-directed CAR for a range of B-cell mediated cancers and autoimmune diseases - where SANA highlighted deep B-cell depletion and immune reset achieved in non-human primates with no off-target toxicity (supporting a potentially best-in-class profile), and management expects an IND/Ph1 start for SG293 in B-cell cancers and B-cell mediated autoimmune diseases by 2027 (but noted the potential for an accelerated timeline with initial data in 2026).
Sionna Therapeutics (SION): Ahead of Ph2a proof-of-concept data from NBD1 stabilizer SION-719 in cystic fibrosis as an add-on to VRTX's standard-of-care Trikafta in mid-2026, the discussion focused on: (1) the study's goal of establishing the synergistic and additive benefit of targeting NBD1 and confirming the translatability of SION's preclinical CFHBE assay, which is key to de-risking SION's dual combination approach (Ph1 healthy volunteer data in mid-2026); (2) the bar for success, which managements views as a ≥10mmol/L improvement in sweat chloride given its historical translation to a clinically meaningful lung function benefit; and (3) the forward development strategy — while a dual combination is the prioritized path, management sees the potential to progress SION-719 into later-stage development.
Spyre Therapeutics Inc. (SYRE): Management discussed: 1) SYRE's relationship with Fairmount Funds, and related sister companies, 2) expectations for results from Part A of the SKYLINE study of 15-20% placebo-adjusted remission rates with comparator drugs annualizing at ~$5bn-10bn longer term, and 3) continued clinical progress for the SKYWAY study, with expectations heightened following the expansion of Roche's and Merck's TL1A programs, which SYRE views as validation of its approach.
Recursion Pharmaceuticals (RXRX): RXRX's incoming CEO Najat Khan and CFO Ben Taylor discussed their key priorities: (1) translating insights from the AI-levered Recursion OS platform to differentiated clinical data - most recently, with the positive Ph1/2 REC-4881 data in familial adenomatous polyposis (FAP), where aligning with the FDA on pivotal design/endpoints is the key next step; (2) focused platform investments in high-value areas; and (3) financial discipline, including a high bar for advancement. Management also touched on the oncology programs, noting: REC-617 (CDK7) combination data in ovarian cancer in 1H27; first Ph1 data for REC-1245 (RBM39) in biomarker-enriched solid tumors/lymphoma in 1H26 and REC-3565 (MALT1) in B-cell malignancies in 1H27; and preclinical PI3Kα H1047R inhibitor REC-7735. Overall, we see the narrative changing for RXRX as it enters into late-stage development in FAP, and remain focused on execution on the prioritized programs.
Tyra Biosciences Inc. (TYRA): Management discussed: 1) upcoming Ph2 readouts for dabogratinib ("dabo"; FGFR3 inhibitor) in intermediate risk non-muscle invasive bladder cancer (IR NMIBC; 1H26) and achondroplasia (2H26), 2) for IR NMIBC, expectations for >70% complete responses (CRs) for an oral therapy, with meaningful readthrough from 3-month data and potential for a Ph3 study, and 3) for achondroplasia, the goal to exceed average height velocity (AHV) of approved therapies (e.g., BMRN's Voxzogo) with at least 7cm/year AHV, supporting a multibrand strategy.
Viridian Therapeutics (VRDN): Management discussed: 1) Veli's BLA status, anticipated decision on priority review by YE25, and potential launch as early as mid-2026, 2) VRDN is planning to launch veli in the US on its own and has been working toward an accelerated review timeline, 3) veli's differentiated profile can potentially expand the current market size of Tepezza by ~30%, 4) VRDN's Ph. 3 chronic trial enrolled all CAS scores and expects these results to be included in the label (vs. Tepezza's that lacks chronic results), 5) the bar for VRDN-003 is Tepezza-like efficacy, 6) VRDN believes the market can potentially grow ~2-3x at peak due to veli's and '003's potential to expand use in chronic TED.
Valneva SE (VLS.PA): Key takeaways from our discussion: 1) Phase 3 readout from VALOR trial of VLA15 in Lyme is still expected in 1H26, 2) Valneva reiterated that the aim has always been for VLA15 to deliver more favourable efficacy to that achieved by LYMErix, and 3) management highlighted the importance of an ACIP for VLA15 in the commercial rollout.
Overall, the Catalyst Clinic indicates 2026 will be a data-heavy year for Goldman's small-cap biotech stock universe.
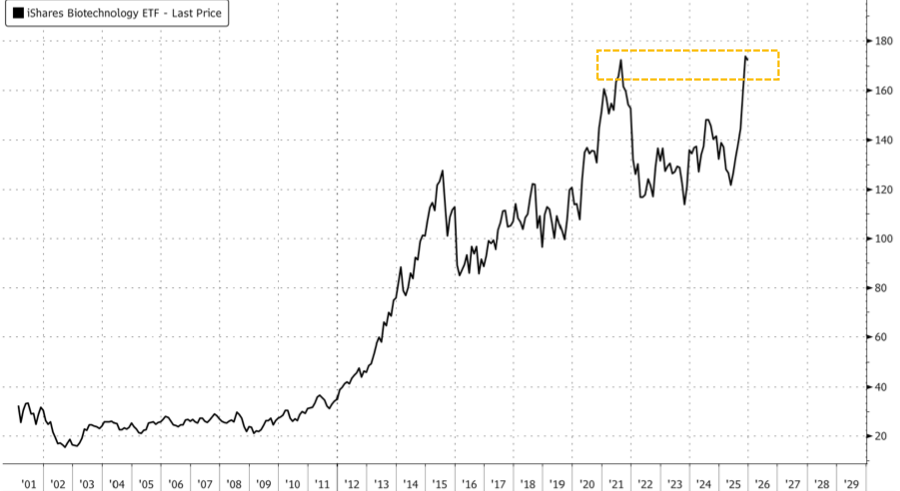
More broadly, the iShares Nasdaq Biotechnology ETF (IBB), the largest and most widely used ETF for broad exposure to U.S. biotechnology companies, has reached new record highs after trading largely sideways since 2021.
https://www.zerohedge.com/markets/goldmans-small-cap-biotech-2026-master-catalyst-list

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.