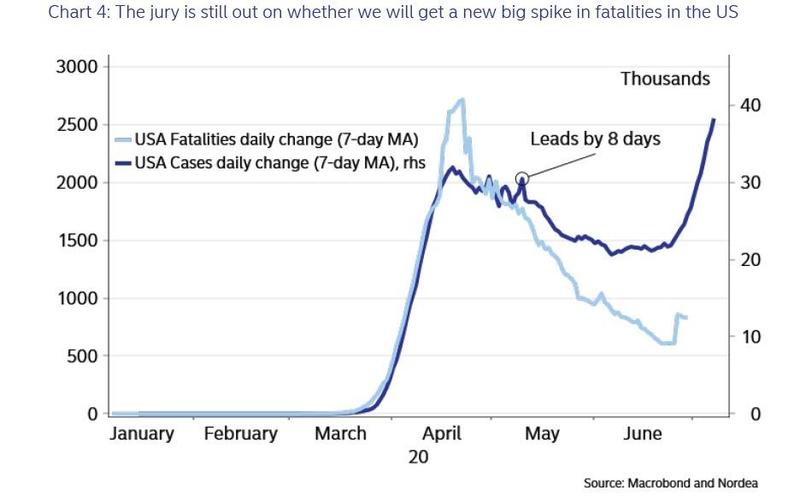
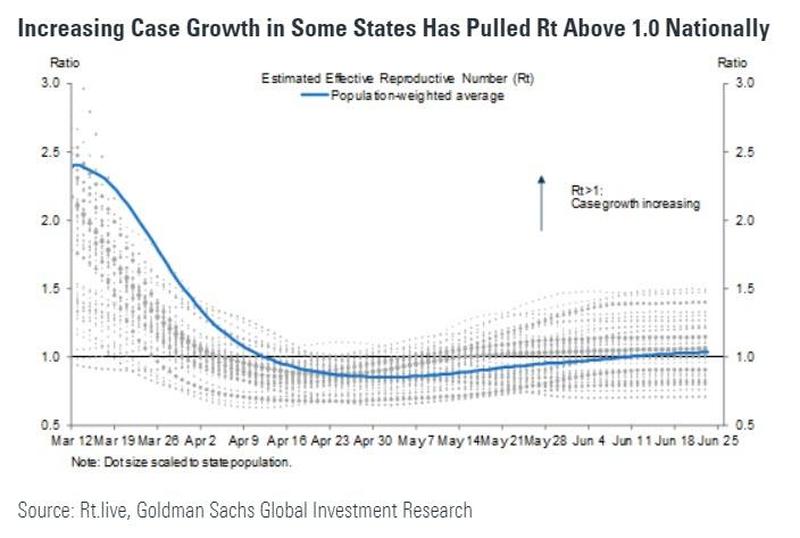
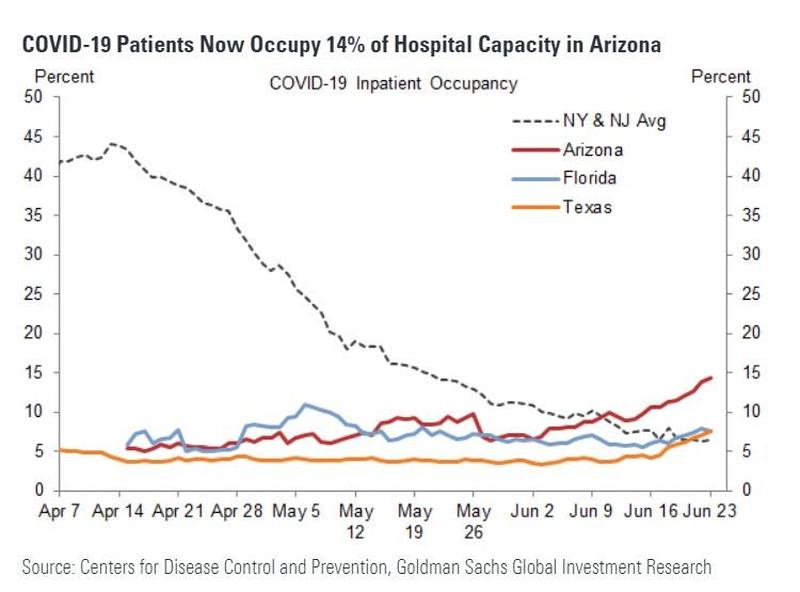
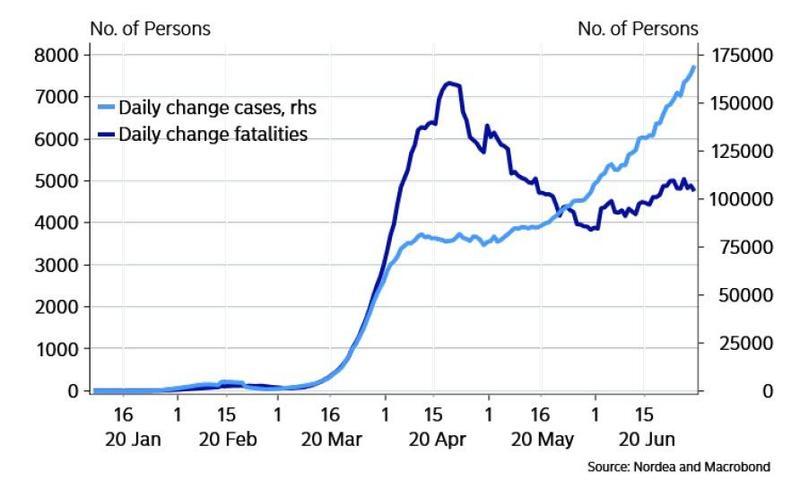
Coronavirus vaccine developers now have some advice from the FDA: To win approval, any vaccine must be at least 50% more effective than placebo in preventing the disease.
FDA Commissioner Stephen Hahn plans to roll out that guidance at a Senate hearing today, the Wall Street Journal reports. It sets a bar about on par with a flu shot’s performance in a good year—but it falls short of some expert recommendations for arresting the virus’ spread.
The agency also won’t approve a shot based on its ability to create antibodies in patients’ blood, the WSJ reports. Experts don’t yet know how those antibodies translate to protection against COVID-19.
Despite the urgency of this particular vaccine hunt, the FDA “will not reduce its standards or cut corners in its review to approve a vaccine,” according to a summary of the guidance cited by the WSJ.
That pledge comes as some industry watchers worry the Trump administration could pressure the agency to approve a vaccine before the election for a political win. Hahn has said politics will not go into COVID-19 vaccine reviews.
An efficacy figure of 50% would compare somewhat favorably to flu vaccine efficacy in the last decade, which has ranged from 19% to 60% since 2010, according to the CDC. Many childhood vaccines are effective for 85% to 95% of recipients, the World Health Organization says.
Merck’s Everbo, an Ebola vaccine developed in response to a 2014 outbreak, was 100% effective in a “ring” vaccination study. The drugmaker is using the same platform for one of its COVID-19 vaccine programs.
But 50% efficacy falls short of what some researchers have concluded would have been needed to quash the COVID-19 outbreak. In a computer model, a team found that a vaccine would’ve needed to be at least 70% effective to halt the spread of the virus, if it were given to 60% of the population within 90 days of the outbreak starting.
For its part, the WHO has set its own success benchmarks for COVID-19 vaccines, according to GlobalData. The higher benchmark calls for 70% efficacy and a duration of protection for one year, while the lower threshold calls for 50% efficacy for 6 months.
In approving a COVID-19 vaccine, there are two approaches the FDA could take—a full approval or an emergency authorization. The full approval would require about 30,000 participants to enroll in a phase 3 trial, which experts have worried might be difficult to conduct if the outbreak quieted over the summer.
But with cases spiking in numerous states, researchers may be in a better position to recruit patients for massive efficacy studies. The U.S. government’s Operation Warp Speed plans to kick off phase 3 trials of several leading candidates this summer, the WSJ previously reported.
An emergency authorization, meanwhile, would be a quicker process than a full approval, but it’d still require the developer to show proof of efficacy. In any case, after potential approvals, the FDA will require a year-long post-marketing study to track potential risks, WSJ reports.
As of Monday, 17 COVID-19 vaccines are in human testing, and more than 130 are in preclinical research stages, according to the World Health Organization.