As COVID-19 vaccination rates pick up around the world, people have reasonably begun to ask: how much longer will this pandemic last? It’s an issue surrounded with uncertainties. But the once-popular idea that enough people will eventually gain immunity to SARS-CoV-2 to block most transmission — a ‘herd-immunity threshold’ — is starting to look unlikely.
That threshold is generally achievable only with high vaccination rates, and many scientists had thought that once people started being immunized en masse, herd immunity would permit society to return to normal. Most estimates had placed the threshold at 60–70% of the population gaining immunity, either through vaccinations or past exposure to the virus. But as the pandemic enters its second year, the thinking has begun to shift. In February, independent data scientist Youyang Gu changed the name of his popular COVID-19 forecasting model from ‘Path to Herd Immunity’ to ‘Path to Normality’. He said that reaching a herd-immunity threshold was looking unlikely because of factors such as vaccine hesitancy, the emergence of new variants and the delayed arrival of vaccinations for children.
Gu is a data scientist, but his thinking aligns with that of many in the epidemiology community. “We’re moving away from the idea that we’ll hit the herd-immunity threshold and then the pandemic will go away for good,” says epidemiologist Lauren Ancel Meyers, executive director of the University of Texas at Austin COVID-19 Modeling Consortium. This shift reflects the complexities and challenges of the pandemic, and shouldn’t overshadow the fact that vaccination is helping. “The vaccine will mean that the virus will start to dissipate on its own,” Meyers says. But as new variants arise and immunity from infections potentially wanes, “we may find ourselves months or a year down the road still battling the threat, and having to deal with future surges”.
Long-term prospects for the pandemic probably include COVID-19 becoming an endemic disease, much like influenza. But in the near term, scientists are contemplating a new normal that does not include herd immunity. Here are some of the reasons behind this mindset, and what they mean for the next year of the pandemic.
It’s unclear whether vaccines prevent transmission
The key to herd immunity is that, even if a person becomes infected, there are too few susceptible hosts around to maintain transmission — those who have been vaccinated or have already had the infection cannot contract and spread the virus. The COVID-19 vaccines developed by Moderna and Pfizer–BioNTech, for example, are extremely effective at preventing symptomatic disease, but it is still unclear whether they protect people from becoming infected, or from spreading the virus to others. That poses a problem for herd immunity.
“Herd immunity is only relevant if we have a transmission-blocking vaccine. If we don’t, then the only way to get herd immunity in the population is to give everyone the vaccine,” says Shweta Bansal, a mathematical biologist at Georgetown University in Washington DC. Vaccine effectiveness for halting transmission needs to be “pretty darn high” for herd immunity to matter, she says, and at the moment, the data aren’t conclusive. “The Moderna and Pfizer data look quite encouraging,” she says, but exactly how well these and other vaccines stop people from transmitting the virus will have big implications.
A vaccine’s ability to block transmission doesn’t need to be 100% to make a difference. Even 70% effectiveness would be “amazing”, says Samuel Scarpino, a network scientist who studies infectious diseases at Northeastern University in Boston, Massachusetts. But there could still be a substantial amount of virus spread that would make it a lot harder to break transmission chains.
Vaccine roll-out is uneven
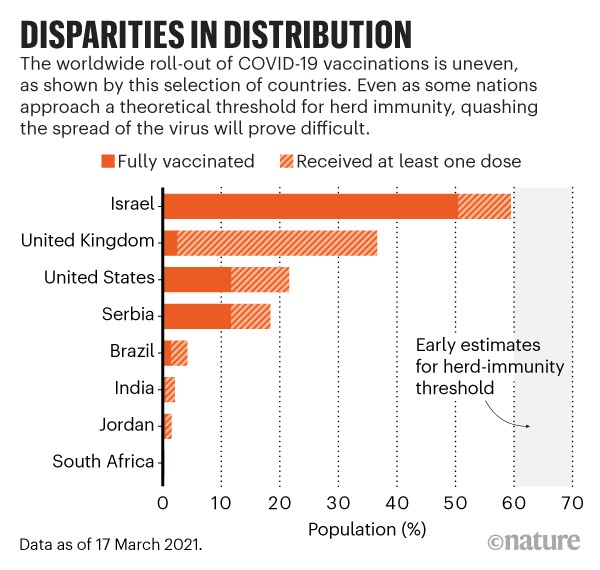
The speed and distribution of vaccine roll-outs matters for various reasons, says Matt Ferrari, an epidemiologist at Pennsylvania State University’s Center for Infectious Disease Dynamics in University Park. A perfectly coordinated global campaign could have wiped out COVID-19, he says, at least theoretically. “It’s a technically feasible thing, but in reality it’s very unlikely that we will achieve that on a global scale,” he says. There are huge variations in the efficiency of vaccine roll-outs between countries (see ‘Disparities in distribution’), and even within them.
Israel began vaccinating its citizens in December 2020, and thanks in part to a deal with Pfizer–BioNTech to share data in exchange for vaccine doses, it currently leads the world in terms of roll-out. Early in the campaign, health workers were vaccinating more than 1% of Israel’s population every day, says Dvir Aran, a biomedical data scientist at the Technion — Israel Institute of Technology in Haifa. As of mid-March, around 50% of the country’s population has been fully vaccinated with the two doses required for protection. “Now the problem is that young people don’t want to get their shots,” Aran says, so local authorities are enticing them with things such as free pizza and beer. Meanwhile, Israel’s neighbours Lebanon, Syria, Jordan and Egypt have yet to vaccinate even 1% of their respective populations.
Across the United States, access to vaccines has been uneven. Some states, such as Georgia and Utah, have fully vaccinated less than 10% of their populations, whereas Alaska and New Mexico have fully vaccinated more than 16%.
In most countries, vaccine distribution is stratified by age, with priority given to older people, who are at the highest risk of dying from COVID-19. When and whether there will be a vaccine approved for children, however, remains to be seen. Pfizer–BioNTech and Moderna have now enrolled teens in clinical trials of their vaccines, and the Oxford–AstraZeneca and Sinovac Biotech vaccines are being tested in children as young as three. But results are still months away. If it’s not possible to vaccinate children, many more adults would need to be immunized to achieve herd immunity, Bansal says. (Those aged 16 and older can receive the Pfizer–BioNTech vaccine, but other vaccines are approved only for ages 18 and up.) In the United States, for example, 24% of people are under 18 years old (according to 2010 census data). If most under-18s can’t receive the vaccine, 100% of over-18s will have to be vaccinated to reach 76% immunity in the population.
Another important thing to consider, Bansal says, is the geographical structure of herd immunity. “No community is an island, and the landscape of immunity that surrounds a community really matters,” she says. COVID-19 has occurred in clusters across the United States as a result of people’s behaviour or local policies. Previous vaccination efforts suggest that uptake will tend to cluster geographically, too, Bansal adds. Localized resistance to the measles vaccination, for example, has resulted in small pockets of disease resurgence. “Geographic clustering is going to make the path to herd immunity a lot less of a straight line, and essentially means we’ll be playing a game of whack-a-mole with COVID outbreaks.” Even for a country with high vaccination rates, such as Israel, if surrounding countries haven’t done the same and populations are able to mix, the potential for new outbreaks remains.
New variants change the herd-immunity equation
Even as vaccine roll-out plans face distribution and allocation hurdles, new variants of SARS-CoV-2 are sprouting up that might be more transmissible and resistant to vaccines. “We’re in a race with the new variants,” says Sara Del Valle, a mathematical and computational epidemiologist at Los Alamos National Laboratory in New Mexico. The longer it takes to stem transmission of the virus, the more time these variants have to emerge and spread, she says.
What’s happening in Brazil offers a cautionary tale. Research published in Science suggests that the slowdown of COVID-19 in the city of Manaus between May and October might have been attributable to herd-immunity effects (L. F. Buss et al. Science 371, 288–292; 2021). The area had been severely hit by the disease, and immunologist Ester Sabino at the University of São Paulo, Brazil, and her colleagues calculated that more than 60% of the population had been infected by June 2020. According to some estimates, that should have been enough to get the population to the herd-immunity threshold, but in January Manaus saw a huge resurgence in cases. This spike happened after the emergence of a new variant known as P.1, which suggests that previous infections did not confer broad protection to the virus. “In January, 100% of the cases in Manaus were caused by P.1,” Sabino says. Scarpino suspects that the 60% figure might have been an overestimate. Even so, he says, “You still have resurgence in the face of a high level of immunity.”
There’s another problem to contend with as immunity grows in a population, Ferrari says. Higher rates of immunity can create selective pressure, which would favour variants that are able to infect people who have been immunized. Vaccinating quickly and thoroughly can prevent a new variant from gaining a foothold. But again, the unevenness of vaccine roll-outs creates a challenge, Ferrari says. “You’ve got a fair bit of immunity, but you still have a fair bit of disease, and you’re stuck in the middle.” Vaccines will almost inevitably create new evolutionary pressures that produce variants, which is a good reason to build infrastructure and processes to monitor for them, he adds.
Immunity might not last forever
Calculations for herd immunity consider two sources of individual immunity — vaccines and natural infection. People who have been infected with SARS-CoV-2 seem to develop some immunity to the virus, but how long that lasts remains a question, Bansal says. Given what’s known about other coronaviruses and the preliminary evidence for SARS-CoV-2, it seems that infection-associated immunity wanes over time, so that needs to be factored in to calculations. “We’re still lacking conclusive data on waning immunity, but we do know it’s not zero and not 100,” Bansal says.
Modellers won’t be able to count everybody who’s been infected when calculating how close a population has come to the herd-immunity threshold. And they’ll have to account for the fact that the vaccines are not 100% effective. If infection-based immunity lasts only for something like months, that provides a tight deadline for delivering vaccines. It will also be important to understand how long vaccine-based immunity lasts, and whether boosters are necessary over time. For both these reasons, COVID-19 could become like the flu.
Vaccines might change human behaviour
At current vaccination rates, Israel is closing in on the theoretical herd-immunity threshold, Aran says. The problem is that, as more people are vaccinated, they will increase their interactions, and that changes the herd-immunity equation, which relies in part on how many people are being exposed to the virus. “The vaccine is not bulletproof,” he says. Imagine that a vaccine offers 90% protection: “If before the vaccine you met at most one person, and now with vaccines you meet ten people, you’re back to square one.”
The most challenging aspects of modelling COVID-19 are the sociological components, Meyers says. “What we know about human behaviour up until now is really thrown out of the window because we are living in unprecedented times and behaving in unprecedented ways.” Meyers and others are trying to adjust their models on the fly to account for shifts in behaviours such as mask wearing and social distancing.
Non-pharmaceutical interventions will continue to play a crucial part in keeping cases down, Del Valle says. The whole point is to break the transmission path, she says, and limiting social contact and continuing protective behaviours such as masking can help to reduce the spread of new variants while vaccines are rolling out.
But it’s going to be hard to stop people reverting to pre-pandemic behaviour. Texas and some other US state governments are already lifting mask mandates, even though substantial proportions of their populations remain unprotected. It’s frustrating to see people easing off these protective behaviours right now, Scarpino says, because continuing with measures that seem to be working, such as limiting indoor gatherings, could go a long way to helping end the pandemic. The herd-immunity threshold is “not a ‘we’re safe’ threshold, it’s a ‘we’re safer’ threshold”, Scarpino says. Even after the threshold has been passed, isolated outbreaks will still occur.
To understand the additive effects of behaviour and immunity, consider that this flu season has been unusually mild. “Influenza is probably not less transmissible than COVID-19,” Scarpino says. “Almost certainly, the reason why flu did not show up this year is because we typically have about 30% of the population immune because they’ve been infected in previous years, and you get vaccination covering maybe another 30%. So you’re probably sitting at 60% or so immune.” Add mask wearing and social distancing, and “the flu just can’t make it”, Scarpino says. This back-of-the-envelope calculation shows how behaviour can change the equation, and why more people would need to be immunized to attain herd immunity as people stop practising behaviours such as social distancing.
Ending transmission of the virus is one way to return to normal. But another could be preventing severe disease and death, says Stefan Flasche, a vaccine epidemiologist at the London School of Hygiene & Tropical Medicine. Given what is known about COVID-19 so far, “reaching herd immunity through vaccines alone is going to be rather unlikely”, he says. It’s time for more realistic expectations. The vaccine is “an absolutely astonishing development”, but it’s unlikely to completely halt the spread, so we need to think of how we can live with the virus, Flasche says. This isn’t as grim as it might sound. Even without herd immunity, the ability to vaccinate vulnerable people seems to be reducing hospitalizations and deaths from COVID-19. The disease might not disappear any time soon, but its prominence is likely to wane.
Nature 591, 520-522 (2021)