GENFIT (NASDAQ:GNFT) +13% premarket enrolls first patient visit for ELATIVE Phase 3 study evaluating elafibranor in Primary Biliary Cholangitis (PBC), a severe cholestatic liver disease that impacts and gradually destroys the bile ducts, leading to inflammation and scarring in the liver.
The ELATIVE Phase 3 study is expected to enroll ~150 patients to evaluate ((2:1, elafibranor:placebo)) with inadequate response to ursodeoxycholic acid (UDCA) following 52 weeks of treatment. The primary endpoint is to measure the treatment of alkaline phosphatase (ALP) < 1.67 x upper limit of normal (ULN), total bilirubin (TB) ≤ ULN and ALP decrease ≥ 15%.
The secondary endpoints will measure the effect of elafibranor on normalization of ALP and change in pruritus from baseline.
Positive outcomes were announced in 2018 for Phase 2 study in PBC and elafibranor.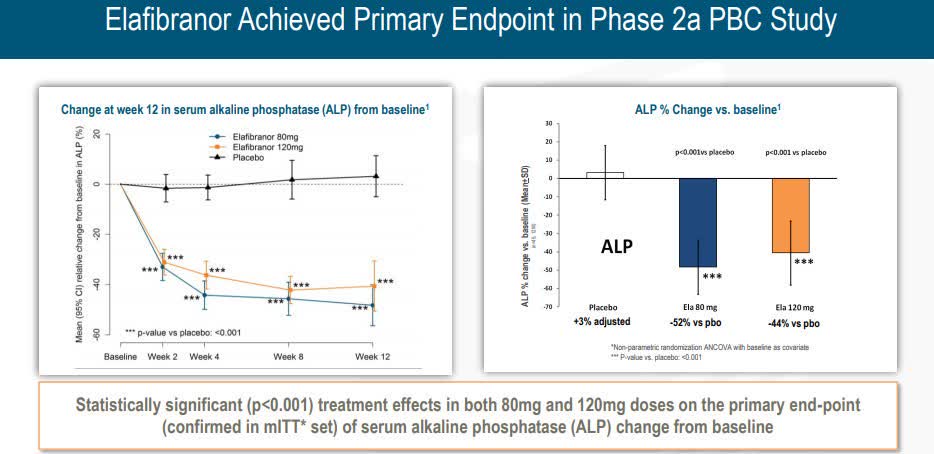
The outcomes in Phase 3 study may potentially be announced at the upcoming Corporate Update on September 30.
Key milestones in pipeline: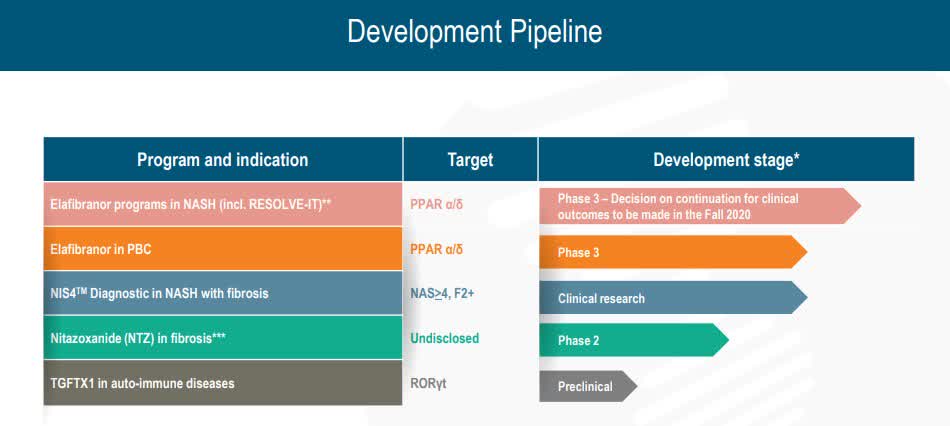 https://seekingalpha.com/news/3617239-first-patient-enrolled-in-genfits-phase-3-elative-study-for-elafibranor-in-pbc-shares-jumps
https://seekingalpha.com/news/3617239-first-patient-enrolled-in-genfits-phase-3-elative-study-for-elafibranor-in-pbc-shares-jumps
No comments:
Post a Comment
Note: Only a member of this blog may post a comment.