Pulse Biosciences, Inc. (Nasdaq: PLSE), a novel bioelectric medicine company commercializing the CellFX® System powered by Nano-Pulse Stimulation™ (NPS™) technology, today announced positive clinical data from an FDA approved Investigational Device Exempt treat and resect study on the use of NPS for low-risk basal cell carcinoma (BCC) lesions. An oral video presentation on the favorable results of performing the CellFX procedure for low-risk superficial and nodular BCC lesion clearance will be delivered at the 2022 American Society for Dermatologic Surgery (ASDS) Annual Meeting, taking place on October 6-10, 2022 in Denver, Colorado.
Search This Blog
Thursday, October 6, 2022
TauRx Deems Phase III Data Sufficient to Send Tau Inhibitor to Regulators
TauRx announced preliminary results Thursday from the Phase III LUCIDITY study, showing hydromethylthionine mesylate (HMTM), an inhibitor of tau aggregates, could slow Alzheimer's disease progression or cognitive decline.
In the overall study sample, 12 months of treatment with HMTM led to an approximately 1-unit improvement in cognition and 1-unit decline in basic functioning scores.
Without any intervention, both metrics are expected to worsen by around five units. HMTM continued to yield cognitive and functional benefits in subgroups of patients with mild cognitive impairment or moderate Alzheimer's disease.
Enrolling nearly 550 patients, LUCIDITY is a double-blinded and placebo-controlled trial that runs for an initial 12 months, followed by another year of a modified delayed-start open-label treatment phase. HMTM is administered as either 16-mg or 8-mg daily doses at first, while all remaining participants will get 16-mg daily treatments during the trial's second phase.
LUCIDITY's co-primary outcomes are cognition and function, as quantified by the Alzheimer's Disease Assessment Scale and the Alzheimer's Disease Cooperative Study-Activities of Daily Living.
Because HMTM is known to affect urine color, LUCIDITY also gave placebo participants a urinary discolorant in the form of methylthioninium chloride, dosed at 4 mg twice weekly. However, patients in this arm showed high blood levels of the active drug, enough to breach the threshold of a clinical effect.
"In the absence of a true placebo, the trial as designed could not determine outcomes on primary clinical endpoints relative to a therapeutically inactive placebo as prespecified," according to a TauRx statement. As a result, LUCIDITY evaluated HMTM's efficacy using historical but closely matched comparators.
Nevertheless, TauRx found its candidate's performance compelling enough.
HMTM significantly slowed cognitive decline and brain atrophy compared to the historical controls, indicative of reduced disease progression, the company stated. Moreover, relative to publicly available placebo arms of various trials in Alzheimer's disease, HMTM conferred meaningful clinical benefits to patients.
HMTM also showed a strong safety profile, with no treatment-related serious adverse events or other amyloid-related imaging anomalies, TauRx reported.
The company plans to use data from LUCIDITY to seek regulatory approval for HMTM in the U.S. and Canada, the filings it expects to submit next year.
A Different Attack on Alzheimer's Disease
While Biogen and Eisai's recent Phase III victory with lecanemab sparked hope for the anti-amyloid approach to treating Alzheimer's disease, experts have urged caution.
HMTM is an oral candidate that targets the other hallmark protein pathology in Alzheimer's disease: tau. Under healthy circumstances, tau proteins help stabilize neurons and facilitate the transport of neurotransmitters and nutrients. When misfolded, tau fibrils clump and tangle, which disrupts healthy neuronal function.
Tau aggregates are known to be markers of cognitive decline and Alzheimer's disease progression. In some cases, these clumps have been detected in brains years before the condition arises. HMTM treats Alzheimer's disease by preventing tau tangles from forming in the first place or by dissolving existing aggregates.
"The field has focused mainly on amyloid as a target for early intervention," said Claude Wischik, executive chairman and co-founder of TauRx, in a statement. "Our data are consistent with the evidence that Tau pathology begins at least 20 years before clinical symptoms appear and is a viable first-line target for treatment."
"The availability of an accessible oral treatment which does not require expensive monitoring over routine clinical care opens up an opportunity to intervene before the onset of the cognitive and functional decline that lead to loss of independence," he added.
National Guard Gives Service Members COVID-19 Vaccine Instead Of Influenza Shot
by Zachary Stieber via The Epoch Times (emphasis ours),
The National Guard administered the COVID-19 vaccine to multiple service members who were lined up for the influenza vaccine, including a member who objected to the COVID-19 vaccine on religious grounds, according to officials and one of the members.
The incident took place in late 2021.
During a vaccination clinic where both flu and COVID-19 vaccines were being administered, “three service members were accidently given a COVID vaccine,” Maj. Carl Lamb, a spokesman for the Maine National Guard, told The Epoch Times in an email.
Mathew Bouchard, who is no longer with the Guard, has identified himself as one of the members.
Bouchard said he was ordered to receive an annual flu vaccine and went to the clinic to get that vaccine. He verified his name, date of birth, and part of his social security number, and told officials at the clinic he was there for the flu vaccine. But he was injected with a dose of a messenger RNA COVID-19 vaccine, officials told him.
“‘You know how you went in for the flu shot? Well, that wasn’t a flu shot. That was a COVID-19 vaccine,'” Bouchard told The Epoch Times, recounting the meeting with superiors.
“I think, in my mind, at that point, it was like, I completely didn’t know if I trusted any people in the military,” he added.
Religious Objections
Each branch of the military ordered members to get vaccinated, after Defense Secretary Lloyd Austin, a Biden appointee, in August of 2021 directed them to do so.
Under federal law, medical and religious exemptions can be sought. The military has been using form letters with striking similarities to deny many religious exemption requests, federal judges have found, and several branches are blocked from taking punitive action against religious objectors.
Bouchard says he was pursuing a religious exemption due to his Christian faith when he was injected with a vaccine against his will. At the time, he only had several months left before his enlistment was over.
Bouchard believes the vaccine mixup was intentional, noting how the military has aggressively tried to pressure members to get vaccinated. The purpose would be to get members to re-enlist, he thinks.
“I think what they did was they thought soldiers who don’t want it and they accidentally get it are just going to come around and continue their service. So I feel like that was a way to vaccinate people,” Bouchard said.
Population Control? Planned Parenthood Encourages Teens To Take Puberty Blockers
Why is Planned Parenthood so interested in trans identity politics when trans people are highly unlikely to have children (only 19%)? It's just another sign that the trans agenda is far more about population reduction that is about personal rights.
A recently unearthed infomercial made by Planned Parenthood last year is marketed directly to teens and promotes puberty blockers as a means to disrupt natural body changes in order to make teens “feel more” like the gender they believe themselves to be psychologically.
In other words, they suggest confused teens fight against their own biology by taking pharmaceuticals which are known to potentially cause chemical castration as well as permanent damage to reproductive processes. This is medically proven to occur, but with leftist politics now poisoning the sciences over the past few years there is a growing narrative that claims puberty blockers are “safe and reversible.”
The claim relies on the use of “unknown quantities,” as there is very little data on the long term effects of puberty blockers in the MAJORITY of people who take them, and in some cases short term usage means POSSIBLE reversal and limited damage to fertility. Advocates for gender affirmation surgeries and hormone therapies will often say that there “is no proof” that puberty blockers are dangerous – This is because of limited studies and data, not because the chemicals and therapies have been proven safe.
In other words, gender activists argue that you can't prove that puberty blockers are NOT safe for everyone. While this is medically disingenuous, it's true that we have no idea what the negative consequences of widespread gender treatments will be 20 years from now. But why take the chance in the first place?
Why allow mentally underdeveloped children with impulsive tendencies to undergo potentially permanent and damaging medical procedures? Why let them destroy themselves in the future just to make them “feel better” today? In the meantime, the data that does exist shows risk of fertility damage depending on length of use.
Furthermore, the primary rationale for the use of puberty blockers and affirmation surgery is to improve the mental health of the individual patient. Yet, we can use the same conditions as the gender activists here by pointing out there there is little proof that such measures actually help the mental health of people with gender dysphoria.
Planned Parenthood from its very creation by elitist Margarate Sanger has made population control its primary mission. As Sanger once stated:
“The most serious evil of our times is that of encouraging the bringing into the world of large families. The most immoral practice of the day is breeding too many children...The most merciful thing that the large family does to one of its infant members is to kill it.”
From “Woman and the New Race,” 1920, Chapter 5: The Wickedness of Creating Large Families
Sanger was an avid supporter of the “Baby Code” in 1934, which would have required married couples to get a permit in order to have a child, and the government would determine if the couple was “fit” to raise a child. Her contentions are recorded in her article “America Needs a Code for Babies,” March 27, 1934, Margaret Sanger Papers, Library of Congress, 128:0312B
The trans agenda along with puberty blockers and affirmation surgeries seem to be a natural extension of the population control goals of the founders of Planned Parenthood. Therefore, it's not at all surprising that they would become so involved in the issue.
Fauci Defends Funding Of EcoHealth, Dismisses Lab Leak Theory
by Eva Fu via The Epoch Times (emphasis ours),
Anthony Fauci, the head of National Institute of Allergy and Infectious Diseases (NIAID), defended his agency’s decision of awarding millions of dollars to an organization that has funded risky virus research in Wuhan.
The New York-based nonprofit, EcoHealth Alliance, received roughly $3 million in grants from the NIAID in late September, the largest annual amount the agency has awarded the group, despite scrutiny over EcoHealth’s partnership with the Wuhan Institute of Virology, the facility that many believe may have started the pandemic.
“If something is peer-reviewed, gets a high recommendation for funding, you can’t arbitrarily decide, ‘I just don’t want to fund it’ because people don’t like them,” Fauci said in an Oct. 4 virtual webinar hosted by the University of South California’s Center for Health Journalism. “If they ever brought that to court, they could sue us and win that in a microsecond. So you’ve got to be careful.”
The new grants came just weeks after the National Institutes of Health (NIH), to which NIAID belongs, terminated an Ecohealth sub-award to the Wuhan lab. The NIH told the House Oversight Committee that the Chinese facility had twice declined their requests to hand over laboratory records so that they could review the research.
Fending Off Criticism
The Wuhan facility, during the 2018 to 2019 grant period, conducted experiments that made bat coronaviruses more lethal, which some experts said met the definition of gain-of-function research—experiments that make a virus more deadly or infectious.
Asked why he was confident that “EcoHealth is a good funding partner” despite criticism about its lack of transparency over virus research, Fauci insisted that the two were separate matters.
“It’s kind of like saying that there is a grant from an institution in the United States, that something really bad about that grant, and therefore, you shouldn’t give any funding to any other element of that institution,” he said.
“You’ve got to be fair, and you’ve got to go by process, not arbitrarily deciding whether you want to fund something or not,” he added.
The new grants, though, have drawn heavy backlash from Republican lawmakers who indicated a COVID-19 origin probe would be a key priority if they win control of the House or Senate after this midterm election.
In light of the new NIH grant, Sen. Joni Ernst (R-Iowa) on Sept. 29 introduced a bill that seeks to stop federal funding to EcoHealth.
“Giving taxpayer money to EcoHealth to study pandemic prevention is like paying a suspected arsonist to conduct fire safety inspections,” she earlier told The Epoch Times. Rep. Cathy McMorris Rodgers (R-Wash.), ranking member of the House Committee on Energy and Commerce, meanwhile, called it “madness.”
“EcoHealth Alliance and Peter Daszak should not be getting a dime of taxpayer funds until they are completely transparent. Period,” she said in a statement.
Fauci, who is retiring in December, said he would have “no problem” giving more testimony to a Republican-controlled Congress.
“I am a big believer in oversight,” he said.
Lab Leak Theory
Emails show that Fauci had sought to play down the lab leak theory in the early period of the pandemic. In April 2020, he told then-NIH director Francis Collins that the hypothesis is “a shiny object” that would go away in time.
Fauci, at the event, stood by his comments in the email, saying that he didn’t think he misjudged the public’s ongoing interest in the lab leak theory.
“There’s always the concern, and I have kept a completely open mind about the possibility that there may have been a lab leak,” but the “lab leak is a theory with no evidence whatsoever,” Fauci said.
https://www.zerohedge.com/covid-19/fauci-defends-funding-ecohealth-dismisses-lab-leak-theory
Credit Suisse Considering Outside Investor, IB Spinoff
The ongoing - and potentially systemically threatening - saga of Credit Suisse took another turn this morning with Bloomberg reporting that the embattled bank is trying to bring in an outside investor to inject money into a spinoff of its advisory and investment banking businesses.
According to people with knowledge of the deliberations, Bloomberg says that talks on reviving the First Boston name for the spun-out businesses, which would get most of their revenue from the US, are also advancing.
A spinout of the dealmaking and underwriting unit would effectively break the troubled division into three pieces, with Credit Suisse keeping a shrunken trading unit while hiving off its securitized products group and other assets it wants to offload. And attracting outside investors would help answer how it will finance a major restructuring, questions that have weighed on the stock as the firm wants to avoid a share sale with the price near record lows.
Additionally, CS stock is up in the pre-market after JPMorgan upgraded to neutral from underweight, saying it sees $15b as a minimum value for the lender.
At the current valuation of 0.27x tangible book value and an $11b market cap, Credit Suisse “should look at every option available to avoid a significantly dilutive capital increase,” JPMorgan analyst Kian Abouhossein wrote, adding that he doesn’t see a capital increase as a given outcome. Instead he sees two scenarios, both of which would lead to material long-term revaluation: Either,
1) the lender does a decisive restructuring of its investment banking business and focuses on being more of an asset gathering geared entity, or
2) it does a ‘half-hearted’ IB restructuring, in which case M&A speculation would increase.
For now, expect shares to remain volatile and headline driven.
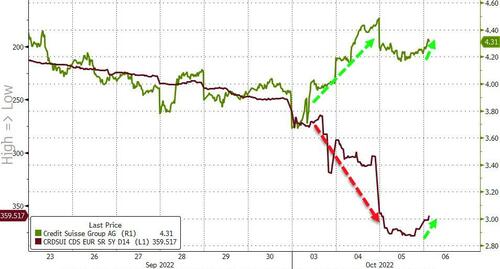
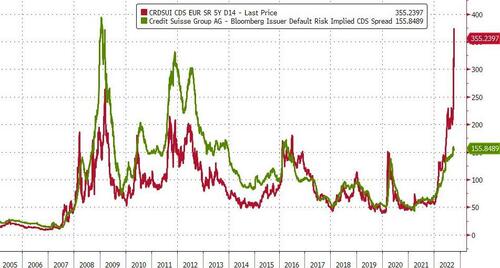
While both CDS and stock prices are improving in the pre-market (lower spread and higher price respectively), there is still a considerable decoupling between the two this week...
Bigger picture, the equity market (green line below) is far more optimistic about the future state of CS than the credit market (red line)...
We look forward to another reassuring letter from the CEO this weekend.
https://www.zerohedge.com/markets/credit-suisse-considering-outside-investor-ib-spinoff
Legal cannabis poses a quandary for US companies screening staff for drugs
Workers at Wyatt Bassett's furniture factory in Virginia use powerful tools to churn out the company's trademark dressers and headboards, so screening new hires for drugs is a no-brainer.
Or it used to be.
Virginia last year fully legalized marijuana -- the first state in the South to do so. The upshot is that "being positive for cannabis does not necessarily disqualify you for employment," said Bassett, CEO of Vaughan-Bassett Furniture Co., which has 575 employees.
Faced with a shortfall in applicants, employers across the U.S. are balancing pressure to ease up on testing for a legal drug with concerns that this could impact safety and raise issues of liability.
The U.S. jobless rate ticked up to 3.7% last month, but it remains near a five-decade low.
"With the war for talent and the labor shortage, especially in some lower paying jobs, it's tough to find and retain folks -- so many are deciding to not test, except for safety sensitive jobs," said Julie Schweber, a senior knowledge adviser at the Society for Human Resource Management. Companies with multiple operations in different parts of the country face an added challenge, she said, because laws differ from state to state.
The challenge of balancing workplace safety and the growing prevalence -- and legalization -- of some types of drugs is especially acute for manufacturers and others who use dangerous equipment.
Last June, Amazon.com Inc. said positive tests for marijuana use would no longer disqualify people from jobs that are not regulated by the U.S. Department of Transportation, such as truck drivers.
The e-commerce giant -- like many other employers -- said it will treat cannabis like alcohol, even though traces of its use linger in the human body far longer and can show up on some types of tests after a worker is no longer impaired by its use. "We will continue to do impairment checks on the job and will test for all drugs and alcohol after any incident," wrote former-CEO Dave Clark, in a blog post at the time of the announcement.
Data from Quest Diagnostics, which handles testing for companies, shows a steady increase in positivity rates for marijuana tests over the past decade -- coinciding with the wave of legalization. In 2012, only 1.9% of workers not subject to federally mandated drug testing requirements failed a pre-employment screening. Last year, that had grown to 4.1%. The jump in positive tests after accidents grew even more during that period, up from 2.4% to 6.7%.
The majority of Fortune 1000 companies have some type of screening in place, but many companies are dropping cannabis tests from the list, said Barry Sample, a senior science consultant who compiles Quest's data. Still, Quest estimates between 30 to 35 million employment-related drug tests are conducted in the U.S. annually.
Most of the tests Quest conducts use urine samples. Other tests rely on swabbing saliva or hair samples. "None of this testing can say whether someone is impaired," said Sample. Rather, the tests will simply indicate the presence of the drug based on a pre-set threshold.
Cannabis use for medical reasons is now legal in 37 states, while recreational use is legal in 19. Quest's data also shows that states that allow recreational use of cannabis have higher positivity rates.
Sample said many employers are shifting screening efforts to focus on drugs that remain illegal and where use in some industries also appears on the upswing. In manufacturing, for instance, Quest found the positivity rate clicked up last year for both methamphetamine and cocaine.
Insurance experts say it is too early to see if the changes will drive up insurance rates for companies that drop testing. "Nobody is going to come out and say, 'We're increasing premiums because you have more stoned workers on the job,'" said Mark Pew, a consultant who specializes in workers' compensation insurance in Georgia. But if, over time, companies that have looser drug screening policies have higher accident rates than those who stick to tougher rules, that could change, he said.
Matt Zender, a senior vice president for workers' compensation strategy with AmTrust Financial Services Inc., said one factor that may obscure or offset the impact of more drug use on the job is the general move toward safer workplaces.
"If you just look at claims per 100 hours of work, overall people are getting injured less often than they were in the past," he said.
Meanwhile, companies continue to fine tune their approaches on the issue. A California manufacturer of plastic bags, contacted by Reuters about their drug screening policies, was surprised to learn that his human resources department was automatically rejecting applicants who test positive for cannabis.
"My nephew would never get a job if I enforced that on him," Kevin Kelly, CEO of Emerald Packaging Inc. in Union City, Calif., said in an email. He said he had now directed his hiring managers to drop the requirement, adding that workers at the factory are not allowed to be impaired on the job. Cannabis use is fully legal in California.