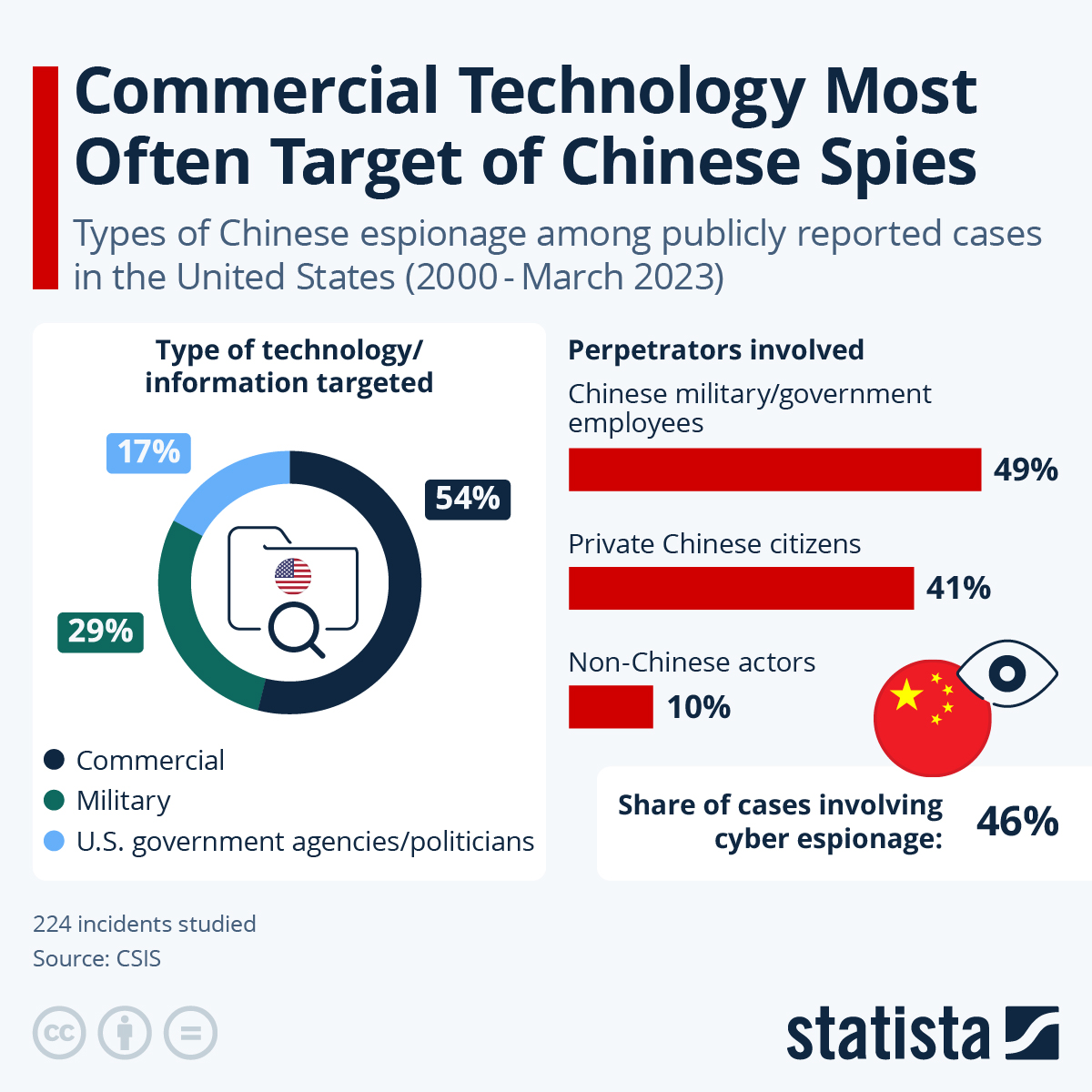
Update (0935ET):
"We write to demand a bipartisan meeting of legislative leaders to end the GOP shutdown of the federal government and decisively address the Republican healthcare crisis. Democrats stand ready to meet with you face to face, anytime and anyplace," Senate Minority Leader Chuck Schumer and Minority Leader Hakeem Jeffries wrote in a letter addressed to President Trump.

This letter comes hours after the Washington Post reported that "a handful" of moderate Senate Democrats are ready to end the record government shutdown.
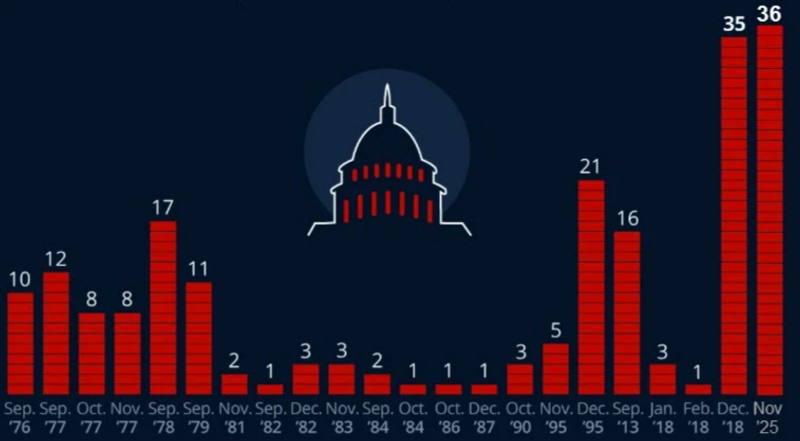
Movement within the Democratic Party to reopen the government comes after last night's election victories, where leftists mostly won, including socialist Zohran Mamdani becoming New York City's next mayor.
Latest Polymarket odds show just a 36% chance the government shutdown ends between Nov. 8 and Nov. 11.
Trump commented earlier...
And noted on Truth Social.
The GOP has learned a lot after last night, especially that the new face of the Dems is the DSA.
* * *
A report overnight suggests that several moderate Senate Democrats are prepared to break ranks and vote to end what has become the longest U.S. government shutdown in history. The push to reopen the government comes conveniently after major victories for Democrats in Tuesday's elections, including socialist Zohran Mamdani becoming New York City's next mayor, and Abigail Spanberger and Mikie Sherrill winning their respective gubernatorial races in Virginia and New Jersey.

The Soros family is very happy.
Washington Post reporters Riley Beggin and Theodoric Meyer cite multiple people familiar with the talks who say "a handful" of moderate Senate Democrats are ready to end the government shutdown.

Here's more color on those conversations:
A bipartisan group of senators is working to craft a deal in which Congress would pass three full-year appropriations bills to fund some agencies, along with a short-term bill that would reopen the rest of the government, according to four people familiar with the talks, who spoke on the condition of anonymity to describe private discussions. In exchange, Senate Republicans would agree to hold a vote at a set date on extending Affordable Care Act subsidies that are otherwise slated to expire. Democrats have insisted throughout the shutdown that the subsidies be extended.
About a dozen Senate Democrats are open to backing the proposal, three of the people estimated, which is still being hammered out — more than enough to break the impasse and reopen the government.
Democrats have used the government shutdown to push for the restoration of taxpayer-funded health insurance subsidies for illegal aliens. That funding stream was terminated when Republicans delivered working families tax cuts that include no tax on tips, overtime, and Social Security as well as major health care reforms earlier this year.
The report continued:
Sen. Gary Peters (D-Michigan), who has been a part of the negotiations, said Tuesday that "everything's on the table," adding that "the pace of talks have increased." Senate Democrats met for lunch Tuesday for nearly three hours to discuss the path forward. Those involved in the talks updated their colleagues on where they stand. Afterward, Peters said it was a "thoughtful" discussion: "It was one of the better caucus meetings I've been in in a while."
Republicans hold a 53-47 majority in the Senate. They need at least eight Democratic votes to overcome a filibuster and reopen the government. Sen. Rand Paul (R-Kentucky) remains opposed to the measure. A couple of lawmakers have already crossed the center aisle, including Sen. Angus King (I-Maine), Sen. Catherine Cortez Masto (D-Nevada), and Sen. John Fetterman (D-Pennsylvania, who support the GOP's clean continuing resolution.
Last week, a Washington Post-ABC News-Ipsos poll found that most respondents blamed President Donald Trump and Republicans more than Democrats for the shutdown than blame Democrats. The informational war to pin the blame on Trump by Democrats, their billionaire funders, their corporate media allies, and their protest industrial complex comes as no surprise, given that this shutdown likely gave Dems a boost during last night's election. This is a firm warning to Republicans, serving as an early indicator of which national strategies will work in the 2026 midterms.
https://www.zerohedge.com/political/democrats-weigh-ending-record-shutdown-conveniently-after-socialist-wins-nyc-mayoral-race