Search This Blog
Monday, December 3, 2018
ResMed to acquire Propeller Health for $225M
ResMed announced it has entered a definitive agreement to acquire Propeller Health, a digital therapeutics company providing connected health solutions for people living with chronic obstructive pulmonary disease and asthma. Propeller helps people and their doctors better manage their COPD and asthma. Propeller’s digital medicine platform consists of small sensors that easily attach to consumers’ inhalers and pair with a mobile app to automatically track medication use and provide personal feedback and insights. Propeller’s clinically validated solutions have demonstrated a 58 percent improvement in medication adherence, 48 percent increase in symptom-free days and 53 percent reduction in emergency room visits. Propeller’s ability to support people in stage II and III severity levels of their COPD are complementary to ResMed’s own suite of cloud-connected ventilators for those with stage III and IV COPD, including Astral, Stellar and AirCurve 10 ST-A with iVAPS – plus ResMed’s new portable oxygen concentrator Mobi. Propeller is privately funded, and based in Madison, Wisconsin, with an office in San Francisco. It will continue to operate as a standalone business within ResMed’s Respiratory Care portfolio. There will be no immediate changes to management, locations or business processes. Van Sickle will continue in his current role, now reporting to McHale. Under the agreement terms, ResMed will acquire Propeller for $225M, which ResMed will fund primarily with its credit facility. Upon closing, the transaction is expected to have a dilutive impact on ResMed’s quarterly non-GAAP earnings per share in the range of 1c to 2c during FY19. ResMed and Propeller expect to finalize the deal before the end of Q3 of ResMed’s FY19, subject to customary closing conditions, including regulatory approvals.
https://thefly.com/landingPageNews.php?id=2831235
Zogenix presents data on Fintepla at Epilepsy Society
Zogenix announced that late-breaking data will be presented on the use of its investigational drug, Fintepla, in children and young adults with Dravet syndrome. These three presentations will include data from the second pivotal Phase 3 trial and long-term efficacy and safety data from a formal interim analysis of the ongoing open-label extension, or OLE, trial. A post-hoc exploration of the clinical meaningfulness of seizure control from the first pivotal Phase 3 trial will also be presented. The data will be presented at the American Epilepsy Society, or AES. Results from Study 1504 will be presented as a follow-up to top-line results that were released in July. Patients in Study 1504 were taking a background anti-epileptic drug medication regimen that included stiripentol and were randomized to placebo or Fintepla 0.5 mg/kg/day. Following a six-week baseline observation period, patients were titrated to their target dose over three weeks and then remained at that fixed dose for 12 weeks. Consistent with Study 1, Study 1504 met the primary endpoint and all key secondary endpoints. Results demonstrated the statistically significant efficacy of Fintepla when added to a stiripentol regimen in children and young adults with Dravet syndrome. Data from Study 1503 will be presented in two posters, one that focuses on effectiveness and overall tolerability of Fintepla and a second on the long-term cardiovascular assessments and observations. A total of 232 patients in Study 1503 were included in the interim analysis of the ongoing OLE trial. The median duration of treatment with Fintepla was 256 days and the range was 58-634 days. A total of 22 patients discontinued treatment. More than 90% of patients remained in the study.
https://thefly.com/landingPageNews.php?id=2831245
https://thefly.com/landingPageNews.php?id=2831245
5 dog food brands recalled this week for problem that can cause kidney ills
“D” might be for “dog,” but too much Vitamin D can cause dog’s kidneys to stop working as they should. The possibility of too much Vitamin D got five brands of dog food from three different manufacturers recalled this week.
Each company-written, FDA-posted recall notice advised dog owners to stop feeding their pooches this particular food. Most can be returned to the place of purchase for a full refund.
“Dogs ingesting elevated levels of Vitamin D may exhibit symptoms such as vomiting, loss of appetite, increased thirst, increased urination, excessive drooling, and weight loss,” one notice explained. “Vitamin D, when consumed at very high levels can lead to serious health issues in dogs including renal dysfunction.”
In layman’s terms, “renal dysfunction” translates to “kidney snafu.”
All have best by dates from Nov. 1, 2018, to Nov. 8, 2019. They went to U.S. retail stores and distributors in Puerto Rico, Japan, Colombia, Israel, Canada and South Korea.
Those with questions can email customer.service@sunshinemills.com or call Sunshine Mills at 800-705-2111 from 8 a.m. to 5 p.m., Eastern time, Monday through Friday.

FDA

FDA

FDA
Any ANF customers with questions can email mwhite@anf.com or call 936-560-5930 from 9 a.m. to 6 p.m., Eastern time, Monday through Friday.
The recalled lots were distributed in Maryland, Delaware, Pennsylvania and New Jersey.
Anyone with questions or wanting to discuss a refund can email customerservice@elmpetfoods.com or call ELM Pet Foods at 1-800-705-2111, 8 a.m. to 5p.m., Eastern time, Monday through Friday.
Biohaven Positive Phase 3 Results for Acute Treatment of Migraine
Biohaven Pharmaceutical Holding Company Ltd. (NYSE: BHVN) today announced positive topline results from a randomized, controlled Phase 3 clinical trial (BHV3000-303 or Study 303) evaluating the efficacy and safety of its Zydis® orally dissolving tablet (ODT) formulation of rimegepant, an oral calcitonin gene-related peptide (CGRP) receptor antagonist, for the acute treatment of migraine. In Study 303, rimegepant Zydis ODT statistically differentiated from placebo on the two co-primary endpoints as well as the first 21 consecutive primary and secondary outcome measures that were prespecified in hierarchical testing. Consistent with the two previous Phase 3 clinical trials, Study 303 met its co-primary registrational endpoints of pain freedom and freedom from most bothersome symptom (MBS) at 2 hours using a single dose (Table 1). Importantly, patients treated with the rimegepant Zydis ODT formulation began to numerically separate from placebo on pain relief as early as 15 minutes, and this difference was statistically significant at 60 minutes (p < 0.0001) (Figure 1). Additionally, a significantly greater percentage of patients treated with rimegepant Zydis ODT returned to normal functioning by 60 minutes as compared to placebo (p = 0.0025). Lasting clinical benefit was observed through 48 hours after a single dose of rimegepant on freedom from pain (p < 0.0001), pain relief (p < 0.0001), freedom from the most bothersome symptom (p = 0.0018), and freedom from functional disability (p < 0.0001). Superiority over placebo was also demonstrated in multiple other secondary endpoints. The vast majority of patients treated with rimegepant Zydis ODT (85%) did not use any rescue medications.
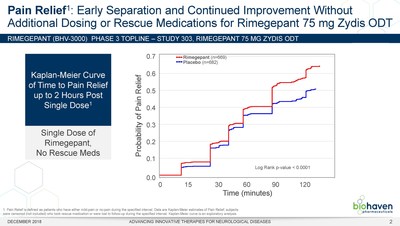
Table 1: Met Co-Primary Endpoints of Pain Freedom & Freedom from Most Bothersome Symptom
2 Hour Endpoint
|
Rimegepant(N=669)
|
Placebo(N=682)
|
Difference
|
p-value
|
Pain Freedom
|
21.2%
|
10.9%
|
10.3%
|
< 0.0001
|
Freedom from MBS1
|
35.1%
|
26.8%
|
8.3%
|
0.0009
|
1. Most Bothersome Symptom of Photophobia, Phonophobia or Nausea
| ||||
The safety and tolerability observations of rimegepant in Study 303 were consistent with the profile previously observed in Studies 301 and 302. Table 2 shows the pooled safety data across all three trials. In study 303, no single adverse event (AE) occurred in the rimegepant group with an incidence higher than 1.6% and overall rates of AEs were similar to placebo. With regard to liver function tests, one patient treated with placebo and one patient treated with rimegepant showed LFTs > 3x ULN in Study 303. Pooled liver function test results across the three pivotal trials (n=3,556) performed to date showed that rimegepant was similar to placebo with regard to aminotransferase (ALT or AST) levels above the upper limit of normal (ULN) and no patients experienced elevations in bilirubin > 2x ULN
Novartis: FDA Priority Review of spinal muscular atrophy med
- The AVXS-101, now known as ZOLGENSMA® (onasemnogene abeparvovec-xxxx)[1], filing is supported by data from the START trial which demonstrated a dramatic increase in survival and transformative improvement in achievement of developmental milestones compared to the natural history of SMA Type 1[2]
- SMA Type 1 is a progressive neuromuscular disease and the leading cause of genetic mortality in infants globally [3]
- ZOLGENSMA® represents the first in a proprietary platform to treat rare, monogenic diseases using gene replacement therapy – technology that replaces a missing or defective gene with a functional copy to correct the underlying cause of genetic disease
Novartis today announced that the U.S. Food and Drug Administration (FDA) has accepted the company’s Biologics License Application (BLA) for AVXS-101, now known as ZOLGENSMA® (onasemnogene abeparvovec-xxxx)[1], an investigational gene replacement therapy for the treatment of spinal muscular atrophy (SMA) Type 1. ZOLGENSMA is designed to address the genetic root cause of SMA Type 1, a deadly neuromuscular disease with limited treatment options. ZOLGENSMA previously received Breakthrough Therapy designation and has been granted Priority Review by the FDA, with regulatory action anticipated in May 2019.
SMA is caused by a defective or missing SMN1 gene.[4] Without a functional SMN1 gene, infants with SMA Type 1 rapidly lose the motor neurons responsible for muscle functions such as breathing, swallowing, speaking and walking.[3] Left untreated, a baby’s muscles become progressively weaker eventually leading to paralysis or death, in most cases by his or her second birthday.[5] Delivered as a single, one-time infusion, this breakthrough technology works by replacing the missing or defective SMN1 gene with a functional copy that makes SMN protein, thereby improving motor neuron function and survival.[2]
“This important step by the FDA brings us ever closer to delivering ZOLGENSMA to patients with SMA Type 1. Babies affected by this rare disease are currently faced with debilitating disease progression and lifelong invasive chronic treatment. As a one-time infusion that addresses the genetic root cause of SMA without the need for repeat dosing, ZOLGENSMA represents a potentially significant therapeutic advance for these patients and their families,” said David Lennon, president of AveXis. “The introduction of one-time, potentially curative therapies will require rethinking how our healthcare system manages diagnosis, treatment, care and associated costs for patients with genetic disease. Novartis and AveXis are proud to lead the way toward a modern healthcare system built on the tremendous value of truly innovative and transformative medicines that could bend the curve of life. We are committed to flexibly partnering with healthcare stakeholders to ensure appropriate access to our medicines.”
Aprea Phase 1b/2 Myelodysplastic Syndromes (MDS) Study at ASH
-95% ORR (by IWG) in 20 evaluable patients
– 70% complete remission (CR) rate in 20 evaluable patients
– No dose-limiting toxicities to date
Aprea Therapeutics presented results at the 2018 ASH Annual Meeting from its Phase Ib/II clinical study in MDS. The ongoing study is evaluating the safety and efficacy of APR-246 in combination with azacitidine for the treatment of TP53 mutated MDS. The study is sponsored by the Moffitt Cancer Center with financial support from the MDS Foundation and the Aplastic Anemia and MDS International Foundation as administrator for the Evans MDS Clinical Research Consortium.
The overall response rate in 20 evaluable patients was 95%, with 14 (70%) patients achieving a complete remission (CR) at data cutoff. Relative to baseline, p53 immunohistochemistry positivity, mutant TP53 variant allele frequency (VAF) and TP53 minimal residual disease (MRD) were significantly decreased at time of disease assessment. No dose-limiting toxicities have been experienced to date and no exacerbation of the expected AZA-related safety profile has been observed.
“The expanding data set from this study is very encouraging,” said David Sallman, M.D., lead principal investigator of the clinical study from the Moffitt Cancer Center. “As of this latest data cutoff, responses have been achieved in nearly all patients, including a 70% complete remission rate, and accompanied by deep molecular remission in the majority of patients as assessed by serial TP53 analysis. In addition, the overall safety experience indicates that the combination regimen of APR-246 and azacitidine is both safe and well-tolerated. Comparison of the current data set to historical AZA clinical experience suggests that combination of APR-246 with AZA may offer these patients a better potential treatment option than AZA alone.”
“The continued positive data from this clinical study has created the potential for a new treatment paradigm for patients with few therapeutic options,” said Christian S. Schade, President and Chief Executive Officer of Aprea. “As a result of this exciting progress, Aprea expects to soon begin enrolling a randomized, controlled Phase III clinical study of APR-246 in combination with AZA for the treatment of TP53 mutated MDS.”
Harpoon Preclinical Data Supports Myeloma Med Development at ASH
Harpoon’s Third Product Candidate is Slated for Clinical Development in 2019
Harpoon Therapeutics, Inc (“Harpoon”), a clinical-stage immunotherapy company developing a novel class of T cell engagers that harness the power of the body’s immune system to treat patients with cancer and other diseases, today presented preclinical data supporting the development of HPN217, a new compound targeting B cell maturation antigen (BCMA). Generated from Harpoon’s Tri-specific T cell Activating Construct (“TriTAC”) platform, HPN217 is engineered to re-direct a patient’s own T cells to kill BCMA-positive cancer cells. HPN217 is Harpoon’s first TriTAC designed for the treatment of hematologic cancers, such as multiple myeloma. Data were presented at the 2018 American Society of Hematology (ASH) Annual Meeting in San Diego, held December 1-4.
“We believe the characteristics of HPN217 – including its structure as a single flexible polypeptide, low molecular weight, and in vitro and in vivo stability with half-life extension – offer advantages over conventional bispecific antibodies targeting BCMA,” said Che-Leung Law, PhD, Harpoon’s Vice President, Translational Medicine. “Supported by preclinical data showing tumor growth inhibition in models of multiple myeloma and mantle cell lymphoma, HPN217 is expected to enter a Phase 1 clinical trial for multiple myeloma in 2019.”
“Recent clinical data has validated the importance of BCMA as a drug target for T cells in the treatment of multiple myeloma,” said Gerald McMahon, PhD, President and Chief Executive Officer of Harpoon. “The introduction of our third TriTAC compound demonstrates the ability of our technology platform to generate new product candidates.”
The poster entitled “Preclinical and Nonclinical Characterization of HPN217: A Tri-specific T cell Activating Construct (TriTAC) Targeting B Cell Maturation Antigen (BCMA) for the Treatment of Multiple Myeloma” can be found on the Publications page of Harpoon’s website.
Subscribe to:
Comments (Atom)